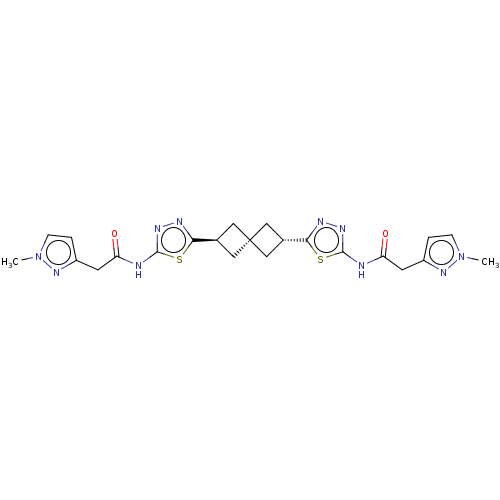
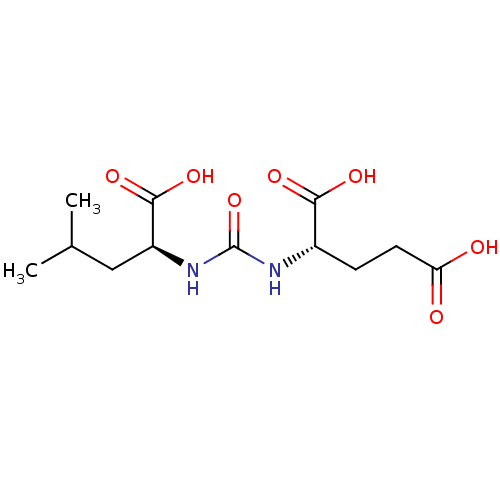
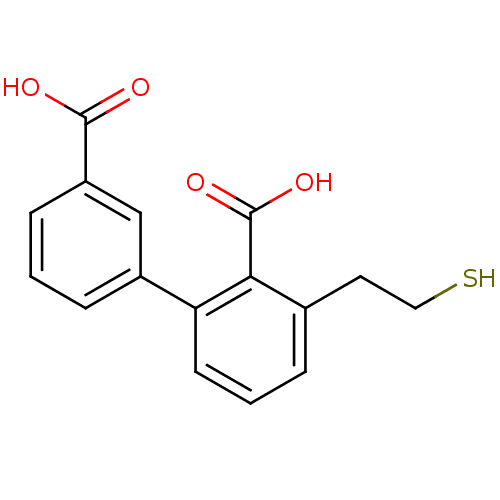
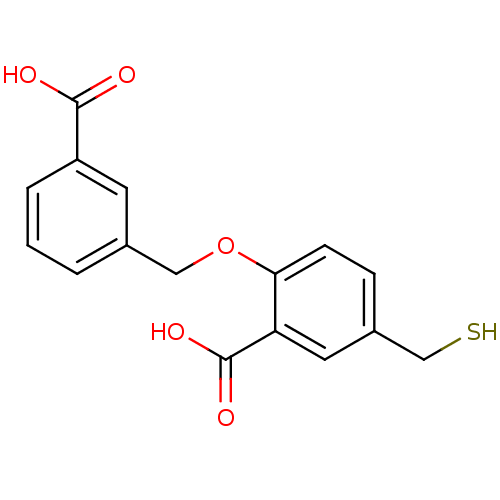
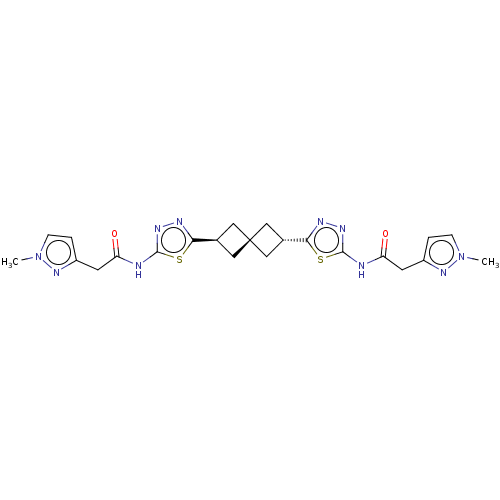
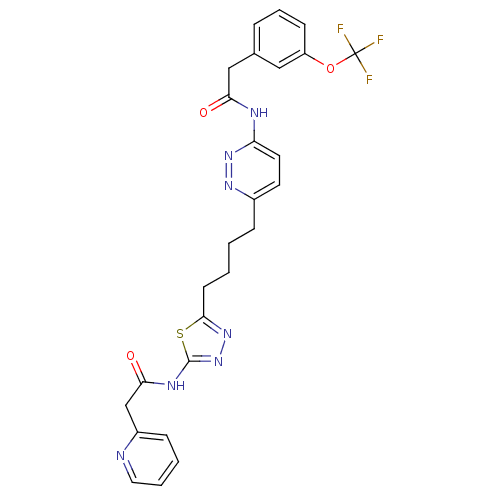
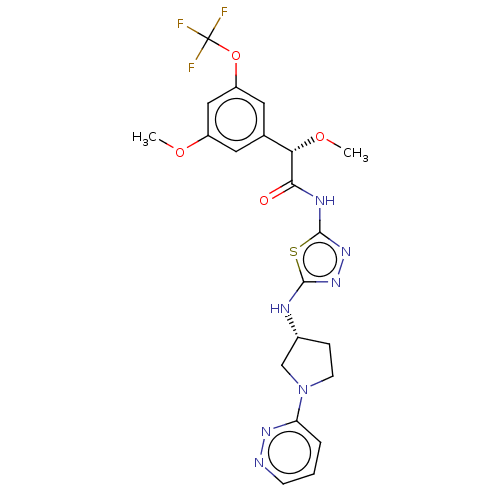
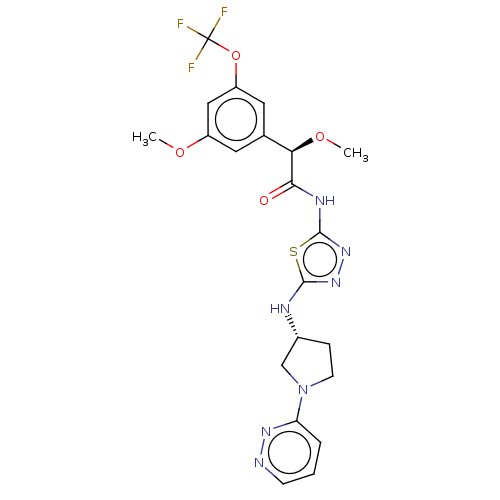
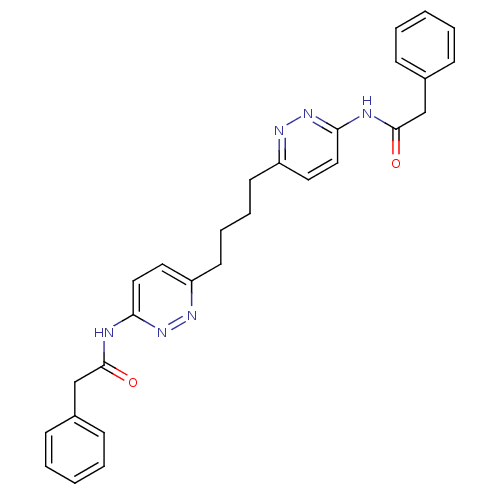
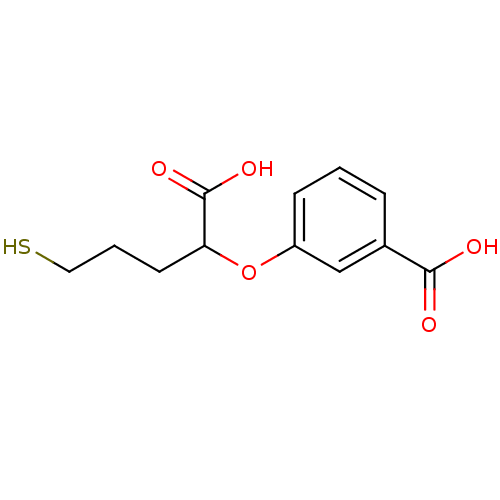
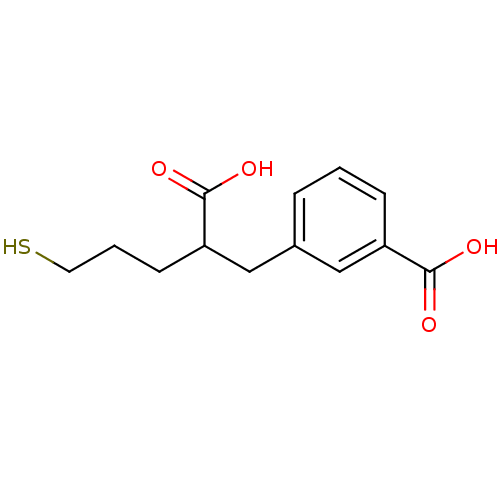
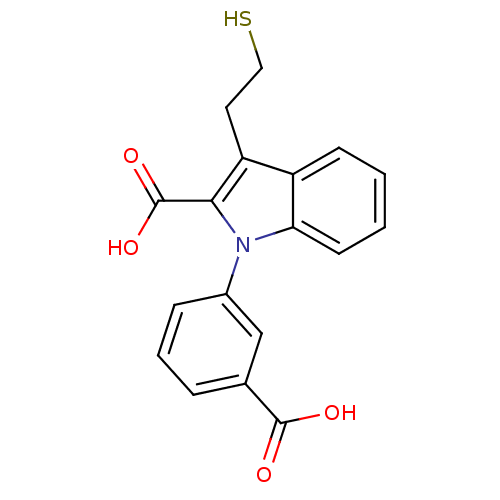
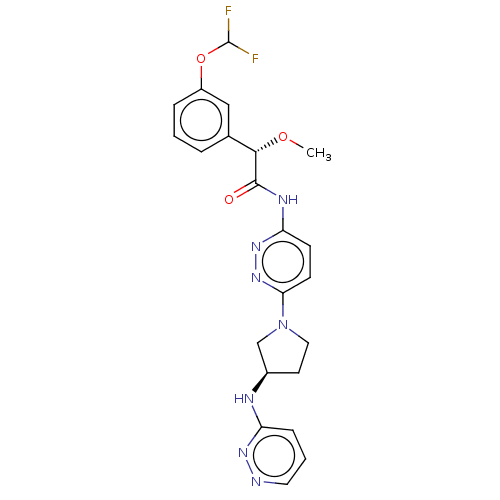
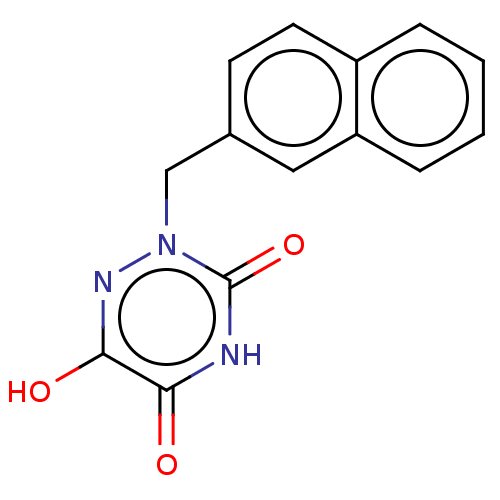
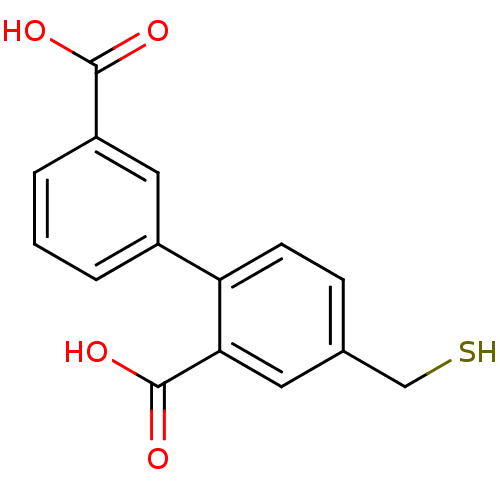
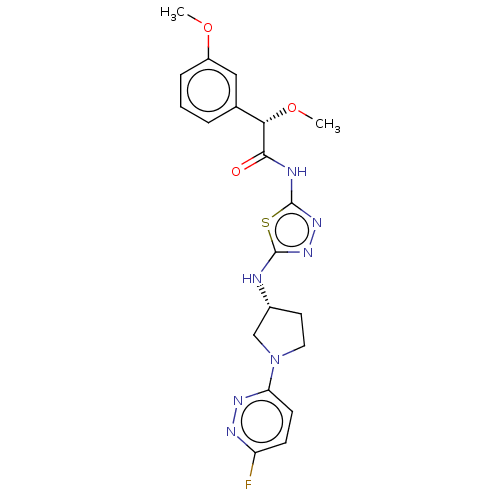
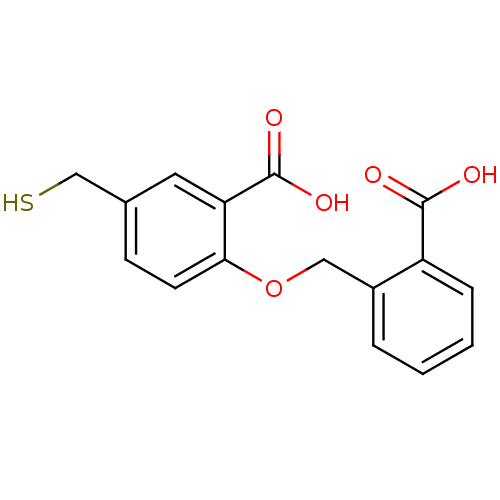
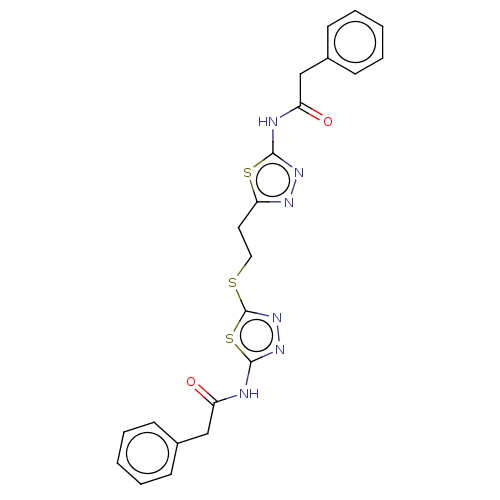
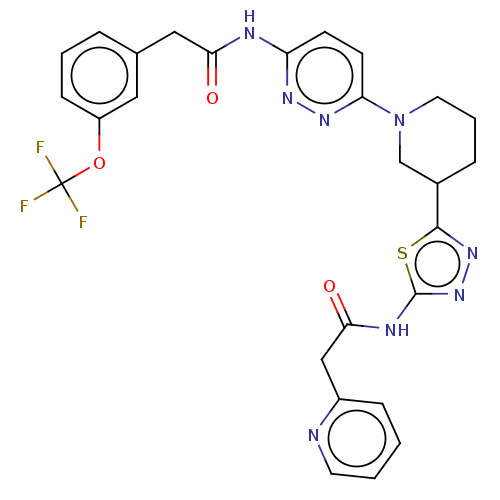
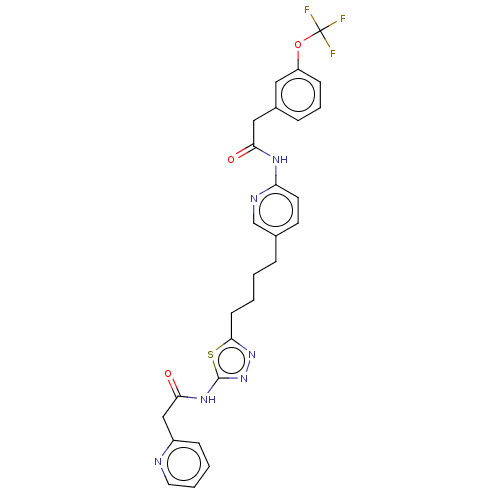
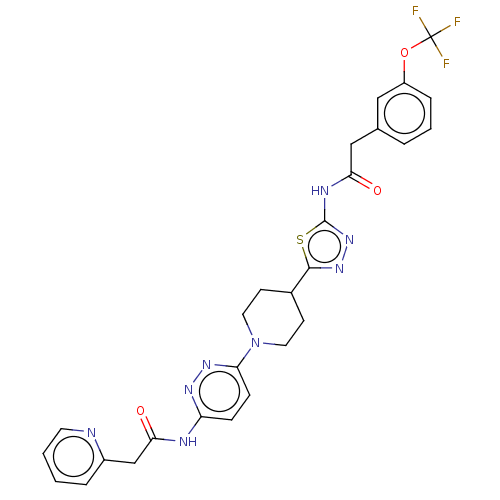
Affinity DataKi: 60nMAssay Description:Competitive inhibition of recombinant human DAAO expressed in HEK cells by double reciprocal plot analysis in presence of D-serineMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataKi: 2.00E+3nMAssay Description:Uncompetitive inhibition of human kidney glutaminase (124 to 669) assessed as reduction of glutamine hydrolysis by double-reciprocal plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.10E+4nMAssay Description:Competitive inhibition of pig kidney DAAO using D-Alanine as substrate by Michaelis-Menten plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 4.00E+4nMAssay Description:Inhibition of human cytidine deaminase by spectrophotometricallyMore data for this Ligand-Target Pair
Affinity DataKi: 4.40E+5nMAssay Description:Inhibition of human cytidine deaminase by spectrophotometricallyMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:Inhibition of kidney-type glutaminase in human BT20 cells assessed as reduction in glutamate levelMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of kidney-type glutaminase in human BT20 cells assessed as reduction in glutamate levelMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMpH: 7.4 T: 2°CAssay Description:GCPII activity in vitro is monitored through the hydrolysis [3H]NAAG to NAA and [3H]Glu. The radioactivity-based assay was miniaturized to a 96-well ...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of kidney-type glutaminase in human BT20 cells assessed as reduction in glutamate levelMore data for this Ligand-Target Pair
TargetGlutamate carboxypeptidase 2(Homo sapiens (Human))
Institute Of Biotechnology Of The Czech Academy Of Sciences
Curated by ChEMBL
Institute Of Biotechnology Of The Czech Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of N-terminally tagged human recombinant GCP2 (44 to 750 residues) extracellular domain expressed in Drosophila melanogaster S2 cells prei...More data for this Ligand-Target Pair
TargetGlutamate carboxypeptidase 2(Homo sapiens (Human))
Institute Of Biotechnology Of The Czech Academy Of Sciences
Curated by ChEMBL
Institute Of Biotechnology Of The Czech Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 0.650nMAssay Description:Inhibition of N-terminally tagged human recombinant GCP2 (44 to 750 residues) extracellular domain expressed in Drosophila melanogaster S2 cells prei...More data for this Ligand-Target Pair
TargetGlutamate carboxypeptidase 2(Homo sapiens (Human))
Institute Of Biotechnology Of The Czech Academy Of Sciences
Curated by ChEMBL
Institute Of Biotechnology Of The Czech Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assayMore data for this Ligand-Target Pair
TargetGlutamate carboxypeptidase 2(Homo sapiens (Human))
Institute Of Biotechnology Of The Czech Academy Of Sciences
Curated by ChEMBL
Institute Of Biotechnology Of The Czech Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assayMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataIC50: 2.70nMAssay Description:Inhibition of kidney-type glutaminase in human BT20 cells assessed as reduction in glutamate levelMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of GAC (unknown origin) assessed as NADH formation using 10 mM glutamine as substrate preincubated for 60 minsMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of human KGA (63 to 669 residues) preincubated for 15 mins using 50 mM glutamine as substrate by resorufin dye based assayMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of human KGA (63 to 669 residues) preincubated for 15 mins using 50 mM glutamine as substrate by resorufin dye based assayMore data for this Ligand-Target Pair
TargetGlutamate carboxypeptidase 2(Homo sapiens (Human))
Institute Of Biotechnology Of The Czech Academy Of Sciences
Curated by ChEMBL
Institute Of Biotechnology Of The Czech Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assayMore data for this Ligand-Target Pair
TargetGlutamate carboxypeptidase 2(Homo sapiens (Human))
Institute Of Biotechnology Of The Czech Academy Of Sciences
Curated by ChEMBL
Institute Of Biotechnology Of The Czech Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 8.90nMAssay Description:Inhibition of N-terminally tagged human recombinant GCP2 (44 to 750 residues) extracellular domain expressed in Drosophila melanogaster S2 cells prei...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibition of GAC (unknown origin) assessed as NADH formation using 10 mM glutamine as substrate preincubated for 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMpH: 7.4 T: 2°CAssay Description:GCPII activity in vitro is monitored through the hydrolysis [3H]NAAG to NAA and [3H]Glu. The radioactivity-based assay was miniaturized to a 96-well ...More data for this Ligand-Target Pair
TargetGlutamate carboxypeptidase 2(Homo sapiens (Human))
Institute Of Biotechnology Of The Czech Academy Of Sciences
Curated by ChEMBL
Institute Of Biotechnology Of The Czech Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assayMore data for this Ligand-Target Pair
Affinity DataIC50: 15nMpH: 7.4 T: 2°CAssay Description:GCPII activity in vitro is monitored through the hydrolysis [3H]NAAG to NAA and [3H]Glu. The radioactivity-based assay was miniaturized to a 96-well ...More data for this Ligand-Target Pair
TargetGlutamate carboxypeptidase 2(Homo sapiens (Human))
Institute Of Biotechnology Of The Czech Academy Of Sciences
Curated by ChEMBL
Institute Of Biotechnology Of The Czech Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Inhibition of N-terminally tagged human recombinant GCP2 (44 to 750 residues) extracellular domain expressed in Drosophila melanogaster S2 cells prei...More data for this Ligand-Target Pair
TargetGlutamate carboxypeptidase 2(Homo sapiens (Human))
Institute Of Biotechnology Of The Czech Academy Of Sciences
Curated by ChEMBL
Institute Of Biotechnology Of The Czech Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assayMore data for this Ligand-Target Pair
TargetGlutamate carboxypeptidase 2(Homo sapiens (Human))
Institute Of Biotechnology Of The Czech Academy Of Sciences
Curated by ChEMBL
Institute Of Biotechnology Of The Czech Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Inhibition of N-terminally tagged human recombinant GCP2 (44 to 750 residues) extracellular domain expressed in Drosophila melanogaster S2 cells prei...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibition of human KGA (63 to 669 residues) preincubated for 15 mins using 50 mM glutamine as substrate by resorufin dye based assayMore data for this Ligand-Target Pair
TargetGlutamate carboxypeptidase 2(Homo sapiens (Human))
Institute Of Biotechnology Of The Czech Academy Of Sciences
Curated by ChEMBL
Institute Of Biotechnology Of The Czech Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 22nMAssay Description:Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assayMore data for this Ligand-Target Pair
Affinity DataIC50: <25nMAssay Description:Inhibition of mouse kidney glutaminase assessed as ammonia formation at 0.1 uM using glutamine as substrate by Nessler's reagent based assay relative...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataIC50: 25nMAssay Description:Inhibition of human KGA (63 to 669 residues) preincubated for 15 mins using 50 mM glutamine as substrate by resorufin dye based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMpH: 8.5 T: 2°CAssay Description:A reliable 96-well plate D-amino acid oxidase (DAAO) assay was developed based on previously published reports (J. Biol. Chem. 277: 27782 (2002)). Br...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMpH: 8.5 T: 2°CAssay Description:A reliable 96-well plate D-amino acid oxidase (DAAO) assay was developed based on previously published reports (J. Biol. Chem. 277: 27782 (2002)). Br...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMpH: 8.5 T: 2°CAssay Description:A reliable 96-well plate D-amino acid oxidase (DAAO) assay was developed based on previously published reports (J. Biol. Chem. 277: 27782 (2002)). Br...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMpH: 8.5 T: 2°CAssay Description:A reliable 96-well plate D-amino acid oxidase (DAAO) assay was developed based on previously published reports (J. Biol. Chem. 277: 27782 (2002)). Br...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataIC50: 30nMAssay Description:Allosteric inhibition of human kidney glutaminase using [3H]-Glutamine as substrate in presence of inhibitor incubated for 45 mins by Perkin Elmer ba...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of recombinant human DAAO assessed as oxidative deamination of D-serine in presence of molecular oxygen and FAD after 20 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of recombinant human DAAO assessed as oxidative deamination of D-serine in presence of molecular oxygen and FAD after 20 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 32nMpH: 7.4 T: 2°CAssay Description:GCPII activity in vitro is monitored through the hydrolysis [3H]NAAG to NAA and [3H]Glu. The radioactivity-based assay was miniaturized to a 96-well ...More data for this Ligand-Target Pair
TargetGlutamate carboxypeptidase 2(Homo sapiens (Human))
Institute Of Biotechnology Of The Czech Academy Of Sciences
Curated by ChEMBL
Institute Of Biotechnology Of The Czech Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 38nMAssay Description:Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assayMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMpH: 8.5 T: 2°CAssay Description:A reliable 96-well plate D-amino acid oxidase (DAAO) assay was developed based on previously published reports (J. Biol. Chem. 277: 27782 (2002)). Br...More data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibition of recombinant human DAAO expressed in HEK cells using D-serine as substrate assessed as formation of alpha-keto acid, ammonia, hydrogen p...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Inhibition of human KGA (63 to 669 residues) preincubated for 15 mins using 50 mM glutamine as substrate by resorufin dye based assayMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Inhibition of human KGA (63 to 669 residues) preincubated for 15 mins using 50 mM glutamine as substrate by resorufin dye based assayMore data for this Ligand-Target Pair
TargetGlutamate carboxypeptidase 2(Homo sapiens (Human))
Institute Of Biotechnology Of The Czech Academy Of Sciences
Curated by ChEMBL
Institute Of Biotechnology Of The Czech Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 41nMAssay Description:Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assayMore data for this Ligand-Target Pair
Affinity DataIC50: 50nMpH: 8.5 T: 2°CAssay Description:A reliable 96-well plate D-amino acid oxidase (DAAO) assay was developed based on previously published reports (J. Biol. Chem. 277: 27782 (2002)). Br...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Allosteric inhibition of human kidney glutaminase using [3H]-Glutamine as substrate in presence of inhibitor incubated for 45 mins by Perkin Elmer ba...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Allosteric inhibition of human kidney glutaminase using [3H]-Glutamine as substrate in presence of inhibitor incubated for 45 mins by Perkin Elmer ba...More data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition of mouse kidney glutaminase assessed as ammonia formation at 0.1 uM using glutamine as substrate by Nessler's reagent based assay relative...More data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition of mouse kidney glutaminase assessed as ammonia formation using glutamine as substrate by Nessler's reagent based assayMore data for this Ligand-Target Pair
Affinity DataIC50: <50nMAssay Description:Inhibition of mouse kidney glutaminase assessed as ammonia formation at 0.1 uM using glutamine as substrate by Nessler's reagent based assay relative...More data for this Ligand-Target Pair


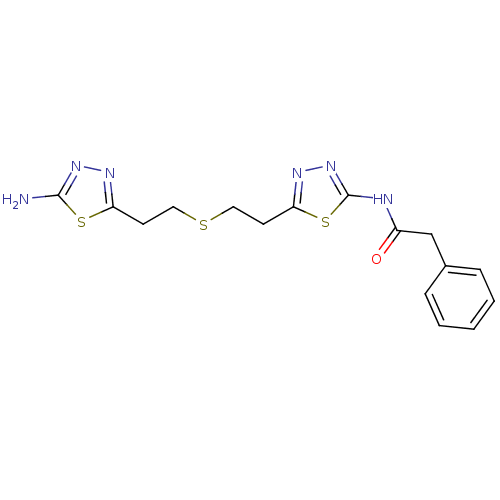
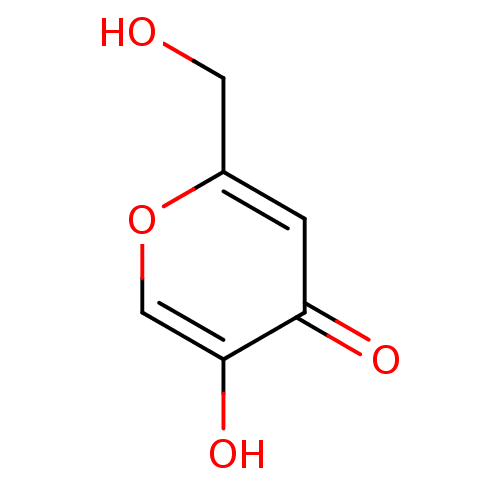
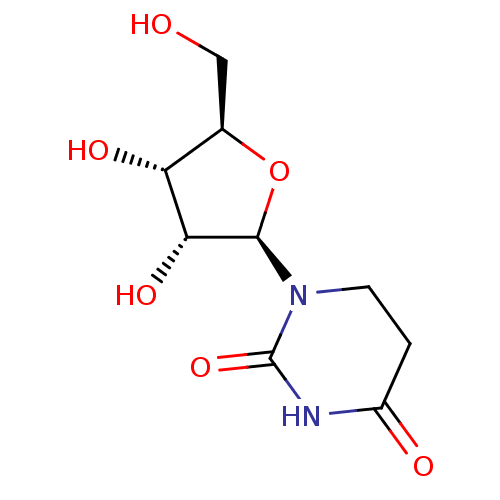

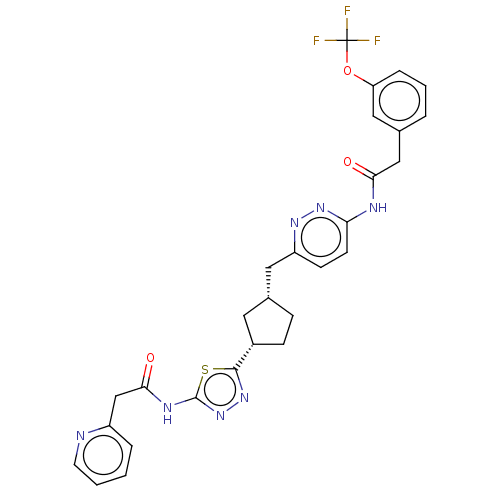
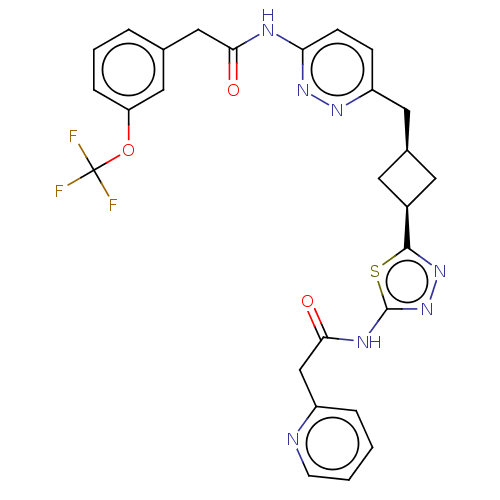
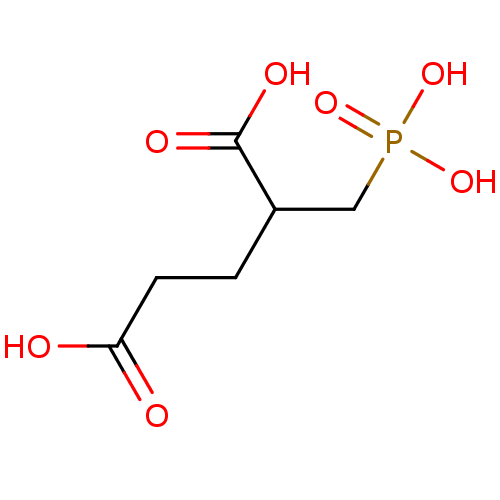
 3D Structure (crystal)
3D Structure (crystal)