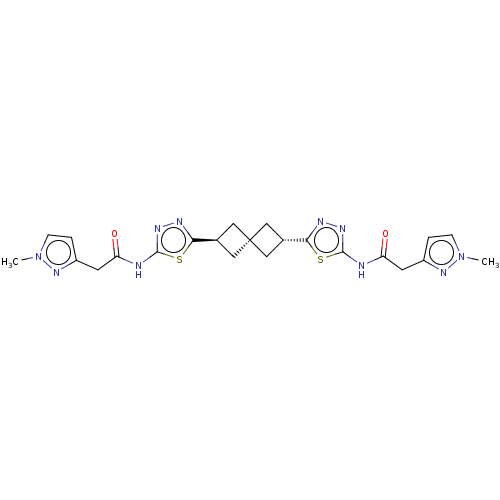
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Glutaminase kidney isoform, mitochondrial
Ligand
BDBM50503313
Substrate
n/a
Meas. Tech.
ChEMBL_1809974 (CHEMBL4309434)
IC50
0.500000±n/a nM
Citation
 Zimmermann, SC; Duvall, B; Tsukamoto, T Recent Progress in the Discovery of Allosteric Inhibitors of Kidney-Type Glutaminase. J Med Chem 62:46-59 (2019) [PubMed] Article
Zimmermann, SC; Duvall, B; Tsukamoto, T Recent Progress in the Discovery of Allosteric Inhibitors of Kidney-Type Glutaminase. J Med Chem 62:46-59 (2019) [PubMed] Article More Info.:
Target
Name:
Glutaminase kidney isoform, mitochondrial
Synonyms:
GLS | GLS1 | GLSK_HUMAN | Glutaminase 1 | K-glutaminase | KIAA0838 | L-glutamine amidohydrolase
Type:
Protein
Mol. Mass.:
73471.89
Organism:
Homo sapiens (Human)
Description:
O94925
Residue:
669
Sequence:
MMRLRGSGMLRDLLLRSPAGVSATLRRAQPLVTLCRRPRGGGRPAAGPAAAARLHPWWGGGGWPAEPLARGLSSSPSEILQELGKGSTHPQPGVSPPAAPAAPGPKDGPGETDAFGNSEGKELVASGENKIKQGLLPSLEDLLFYTIAEGQEKIPVHKFITALKSTGLRTSDPRLKECMDMLRLTLQTTSDGVMLDKDLFKKCVQSNIVLLTQAFRRKFVIPDFMSFTSHIDELYESAKKQSGGKVADYIPQLAKFSPDLWGVSVCTVDGQRHSTGDTKVPFCLQSCVKPLKYAIAVNDLGTEYVHRYVGKEPSGLRFNKLFLNEDDKPHNPMVNAGAIVVTSLIKQGVNNAEKFDYVMQFLNKMAGNEYVGFSNATFQSERESGDRNFAIGYYLKEKKCFPEGTDMVGILDFYFQLCSIEVTCESASVMAATLANGGFCPITGERVLSPEAVRNTLSLMHSCGMYDFSGQFAFHVGLPAKSGVAGGILLVVPNVMGMMCWSPPLDKMGNSVKGIHFCHDLVSLCNFHNYDNLRHFAKKLDPRREGGDQRVKSVINLLFAAYTGDVSALRRFALSAMDMEQRDYDSRTALHVAAAEGHVEVVKFLLEACKVNPFPKDRWNNTPMDEALHFGHHDVFKILQEYQVQYTPQGDSDNGKENQTVHKNLDGLL
Inhibitor
Name:
BDBM50503313
Synonyms:
CHEMBL4454263
Type:
Small organic molecule
Emp. Form.:
C23H26N10O2S2
Mol. Mass.:
538.648
SMILES:
Cn1ccc(CC(=O)Nc2nnc(s2)[C@H]2C[C@]3(C[C@@H](C3)c3nnc(NC(=O)Cc4ccn(C)n4)s3)C2)n1 |r,wU:16.17,18.21,wD:14.14,(22.28,-3.9,;23.81,-3.75,;24.59,-2.42,;26.09,-2.75,;26.25,-4.29,;27.58,-5.07,;28.91,-4.31,;28.93,-2.77,;30.24,-5.09,;31.58,-4.33,;32.06,-2.86,;33.6,-2.86,;34.08,-4.33,;32.83,-5.23,;35.41,-5.09,;35.8,-6.58,;37.29,-6.19,;37.69,-7.68,;39.17,-7.28,;38.78,-5.79,;40.51,-8.05,;41.75,-7.14,;43,-8.05,;42.52,-9.51,;43.84,-10.28,;45.18,-9.52,;45.19,-7.98,;46.51,-10.29,;47.85,-9.53,;48.01,-7.99,;49.52,-7.67,;50.28,-9.01,;51.82,-9.17,;49.25,-10.15,;40.98,-9.51,;36.89,-4.7,;24.83,-4.9,)|