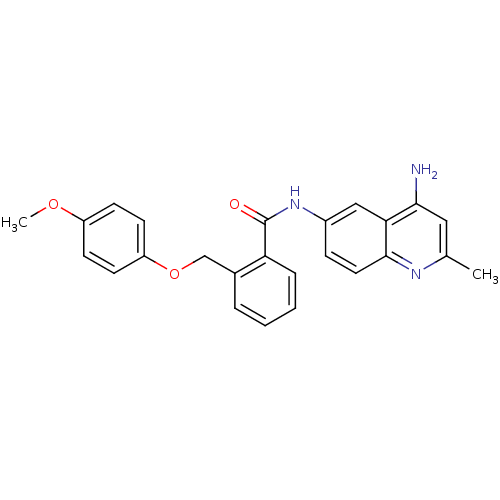
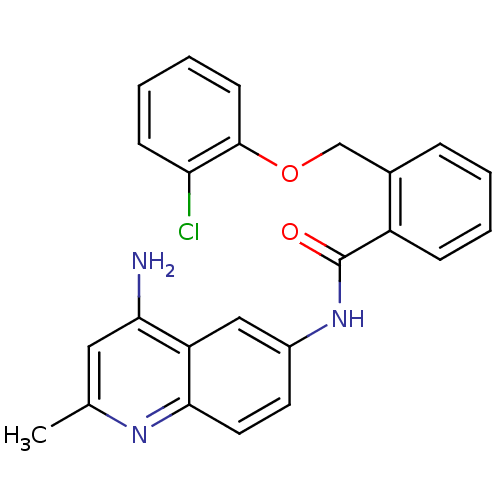
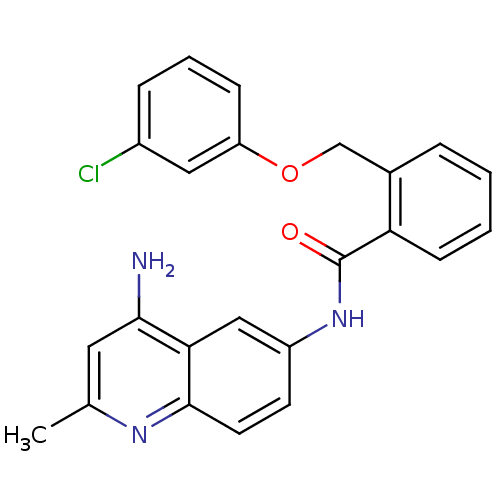
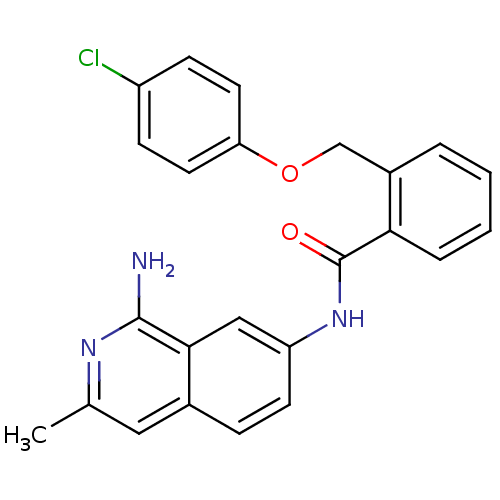
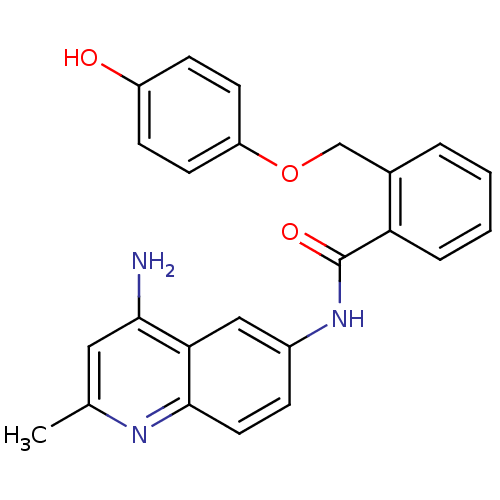
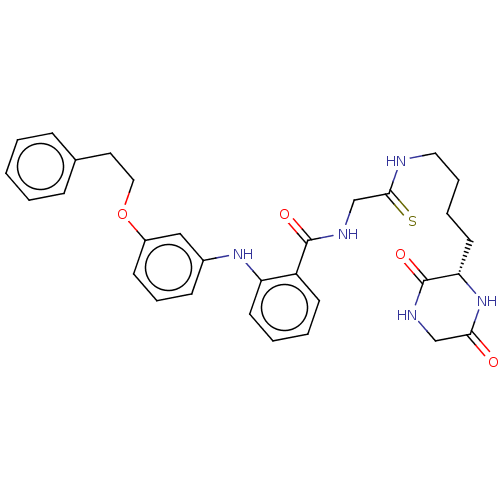
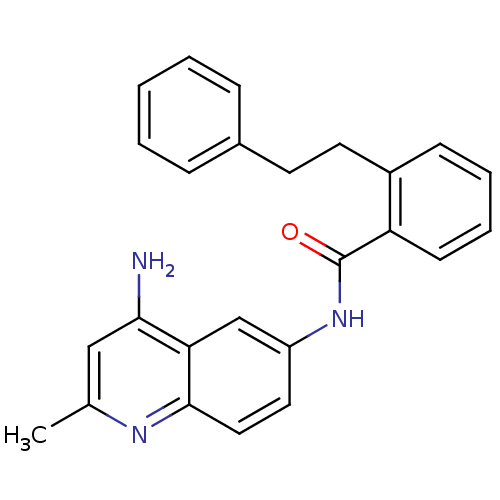
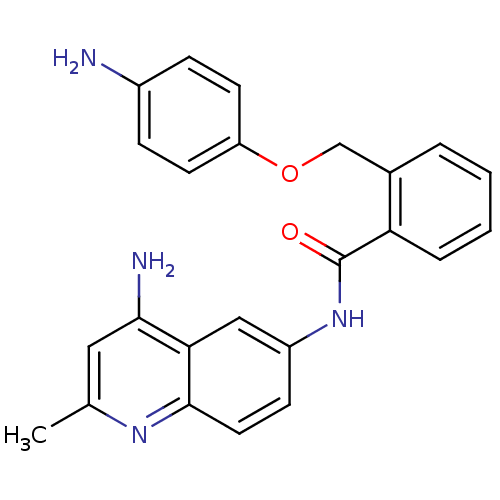
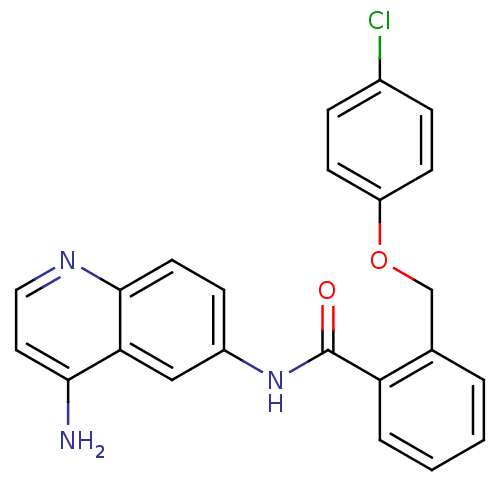
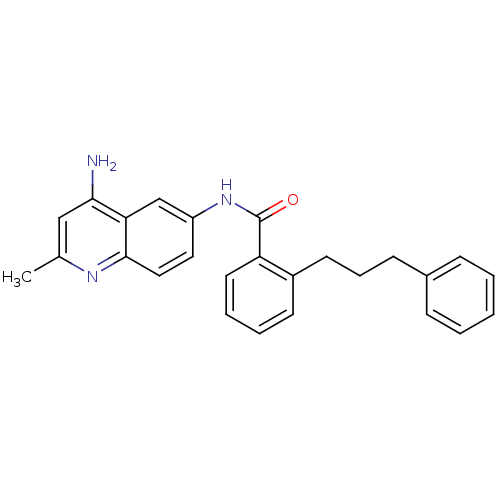
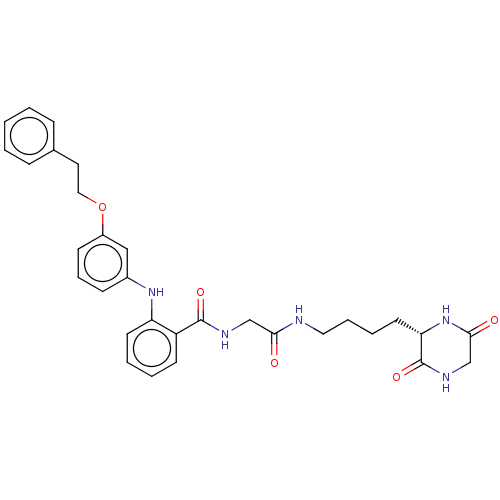
Report error Found 2014 with Last Name = 'iida' and Initial = 't'
Affinity DataKi: 1.80nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2.20nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2.30nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2.60nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataKi: 6.5nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataKi: 7nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataKi: 8.20nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataKi: 37nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataKi: 47nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataKi: 51nMAssay Description:Compound was evaluated for its ability to displace [3H]-nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNAD-dependent protein deacetylase sirtuin-2(Human)
Kyoto Prefectural University of Medicine
Curated by ChEMBL
Kyoto Prefectural University of Medicine
Curated by ChEMBL
Affinity DataKi: 68nMAssay Description:Inhibition of human N-terminal His-tagged SIRT2 expressed in Escherichia coli using p53 (Gln-Pro-Lys-Lys(Ac)) (317 to 320 residues) as substrate incu...More data for this Ligand-Target Pair
Affinity DataKi: 80nMAssay Description:Compound was evaluated for its ability to displace [3H]-nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataKi: 82nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataKi: 86nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataKi: 89nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataKi: 103nMAssay Description:Inhibition of [3H]diprenorphine (0.33 nM) binding from human Opioid receptor mu 1 expressed in CHO-K1 cells.More data for this Ligand-Target Pair
Affinity DataKi: 121nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataKi: 369nMAssay Description:Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetNAD-dependent protein deacetylase sirtuin-2(Human)
Kyoto Prefectural University of Medicine
Curated by ChEMBL
Kyoto Prefectural University of Medicine
Curated by ChEMBL
Affinity DataKi: 470nMAssay Description:Inhibition of human N-terminal His-tagged SIRT2 expressed in Escherichia coli using p53 (Gln-Pro-Lys-Lys(Ac)) (317 to 320 residues) as substrate incu...More data for this Ligand-Target Pair
Affinity DataKi: 1.06E+3nMAssay Description:Inhibition of [3H]naltrindole (0.55 nM) binding from human Opioid receptor kappa 1More data for this Ligand-Target Pair
Affinity DataKi: 8.65E+3nMAssay Description:Inhibition of [3H]naltrindole (0.55 nM) binding from human Opioid receptor delta 1 expressed in CHO-K1 cells.More data for this Ligand-Target Pair
TargetGTPase KRas (G12D) and Son of Sevenless Homolog 1 (SOS)(Human)
Chugai Seiyaku Kabushiki Kaisha
US Patent
Chugai Seiyaku Kabushiki Kaisha
US Patent
Affinity DataIC50: 0.300nMAssay Description:Protein-protein interaction inhibition (PPI) between Kras and SOS1 was measured by energy transfer from nickel-conjugated donor beads to streptavidin...More data for this Ligand-Target Pair
Target InfoGoogleScholar
TargetGTPase KRas (G12D) and Son of Sevenless Homolog 1 (SOS)(Human)
Chugai Seiyaku Kabushiki Kaisha
US Patent
Chugai Seiyaku Kabushiki Kaisha
US Patent
Affinity DataIC50: 0.300nMAssay Description:Protein-protein interaction inhibition (PPI) between Kras and SOS1 was measured by energy transfer from nickel-conjugated donor beads to streptavidin...More data for this Ligand-Target Pair
Target InfoGoogleScholar
TargetGTPase KRas (G12D) and Son of Sevenless Homolog 1 (SOS)(Human)
Chugai Seiyaku Kabushiki Kaisha
US Patent
Chugai Seiyaku Kabushiki Kaisha
US Patent
Affinity DataIC50: 0.330nMAssay Description:Protein-protein interaction inhibition (PPI) between Kras and SOS1 was measured by energy transfer from nickel-conjugated donor beads to streptavidin...More data for this Ligand-Target Pair
Target InfoGoogleScholar
TargetGTPase KRas (G12D) and Son of Sevenless Homolog 1 (SOS)(Human)
Chugai Seiyaku Kabushiki Kaisha
US Patent
Chugai Seiyaku Kabushiki Kaisha
US Patent
Affinity DataIC50: 0.330nMAssay Description:Protein-protein interaction inhibition (PPI) between Kras and SOS1 was measured by energy transfer from nickel-conjugated donor beads to streptavidin...More data for this Ligand-Target Pair
Target InfoGoogleScholar
TargetGTPase KRas (G12D) and Son of Sevenless Homolog 1 (SOS)(Human)
Chugai Seiyaku Kabushiki Kaisha
US Patent
Chugai Seiyaku Kabushiki Kaisha
US Patent
Affinity DataIC50: 0.330nMAssay Description:Protein-protein interaction inhibition (PPI) between Kras and SOS1 was measured by energy transfer from nickel-conjugated donor beads to streptavidin...More data for this Ligand-Target Pair
Target InfoGoogleScholar
TargetGTPase KRas (G12D) and Son of Sevenless Homolog 1 (SOS)(Human)
Chugai Seiyaku Kabushiki Kaisha
US Patent
Chugai Seiyaku Kabushiki Kaisha
US Patent
Affinity DataIC50: 0.330nMAssay Description:Protein-protein interaction inhibition (PPI) between Kras and SOS1 was measured by energy transfer from nickel-conjugated donor beads to streptavidin...More data for this Ligand-Target Pair
Target InfoGoogleScholar
TargetGTPase KRas (G12D) and Son of Sevenless Homolog 1 (SOS)(Human)
Chugai Seiyaku Kabushiki Kaisha
US Patent
Chugai Seiyaku Kabushiki Kaisha
US Patent
Affinity DataIC50: 0.350nMAssay Description:Protein-protein interaction inhibition (PPI) between Kras and SOS1 was measured by energy transfer from nickel-conjugated donor beads to streptavidin...More data for this Ligand-Target Pair
Target InfoGoogleScholar
TargetGTPase KRas (G12D) and Son of Sevenless Homolog 1 (SOS)(Human)
Chugai Seiyaku Kabushiki Kaisha
US Patent
Chugai Seiyaku Kabushiki Kaisha
US Patent
Affinity DataIC50: 0.350nMAssay Description:Protein-protein interaction inhibition (PPI) between Kras and SOS1 was measured by energy transfer from nickel-conjugated donor beads to streptavidin...More data for this Ligand-Target Pair
Target InfoGoogleScholar
TargetGTPase KRas (G12D) and Son of Sevenless Homolog 1 (SOS)(Human)
Chugai Seiyaku Kabushiki Kaisha
US Patent
Chugai Seiyaku Kabushiki Kaisha
US Patent
Affinity DataIC50: 0.350nMAssay Description:Protein-protein interaction inhibition (PPI) between Kras and SOS1 was measured by energy transfer from nickel-conjugated donor beads to streptavidin...More data for this Ligand-Target Pair
Target InfoGoogleScholar
TargetGTPase KRas (G12D) and Son of Sevenless Homolog 1 (SOS)(Human)
Chugai Seiyaku Kabushiki Kaisha
US Patent
Chugai Seiyaku Kabushiki Kaisha
US Patent
Affinity DataIC50: 0.360nMAssay Description:Protein-protein interaction inhibition (PPI) between Kras and SOS1 was measured by energy transfer from nickel-conjugated donor beads to streptavidin...More data for this Ligand-Target Pair
Target InfoGoogleScholar
TargetGTPase KRas (G12D) and Son of Sevenless Homolog 1 (SOS)(Human)
Chugai Seiyaku Kabushiki Kaisha
US Patent
Chugai Seiyaku Kabushiki Kaisha
US Patent
Affinity DataIC50: 0.370nMAssay Description:Protein-protein interaction inhibition (PPI) between Kras and SOS1 was measured by energy transfer from nickel-conjugated donor beads to streptavidin...More data for this Ligand-Target Pair
Target InfoGoogleScholar
TargetGTPase KRas (G12D) and Son of Sevenless Homolog 1 (SOS)(Human)
Chugai Seiyaku Kabushiki Kaisha
US Patent
Chugai Seiyaku Kabushiki Kaisha
US Patent
Affinity DataIC50: 0.370nMAssay Description:Protein-protein interaction inhibition (PPI) between Kras and SOS1 was measured by energy transfer from nickel-conjugated donor beads to streptavidin...More data for this Ligand-Target Pair
Target InfoGoogleScholar
TargetGTPase KRas (G12D) and Son of Sevenless Homolog 1 (SOS)(Human)
Chugai Seiyaku Kabushiki Kaisha
US Patent
Chugai Seiyaku Kabushiki Kaisha
US Patent
Affinity DataIC50: 0.370nMAssay Description:Protein-protein interaction inhibition (PPI) between Kras and SOS1 was measured by energy transfer from nickel-conjugated donor beads to streptavidin...More data for this Ligand-Target Pair
Target InfoGoogleScholar
TargetGTPase KRas (G12D) and Son of Sevenless Homolog 1 (SOS)(Human)
Chugai Seiyaku Kabushiki Kaisha
US Patent
Chugai Seiyaku Kabushiki Kaisha
US Patent
Affinity DataIC50: 0.380nMAssay Description:Protein-protein interaction inhibition (PPI) between Kras and SOS1 was measured by energy transfer from nickel-conjugated donor beads to streptavidin...More data for this Ligand-Target Pair
Target InfoGoogleScholar
TargetGTPase KRas (G12D) and Son of Sevenless Homolog 1 (SOS)(Human)
Chugai Seiyaku Kabushiki Kaisha
US Patent
Chugai Seiyaku Kabushiki Kaisha
US Patent
Affinity DataIC50: 0.380nMAssay Description:Protein-protein interaction inhibition (PPI) between Kras and SOS1 was measured by energy transfer from nickel-conjugated donor beads to streptavidin...More data for this Ligand-Target Pair
Target InfoGoogleScholar
TargetGTPase KRas (G12D) and Son of Sevenless Homolog 1 (SOS)(Human)
Chugai Seiyaku Kabushiki Kaisha
US Patent
Chugai Seiyaku Kabushiki Kaisha
US Patent
Affinity DataIC50: 0.380nMAssay Description:Protein-protein interaction inhibition (PPI) between Kras and SOS1 was measured by energy transfer from nickel-conjugated donor beads to streptavidin...More data for this Ligand-Target Pair
Target InfoGoogleScholar
TargetGTPase KRas (G12D) and Son of Sevenless Homolog 1 (SOS)(Human)
Chugai Seiyaku Kabushiki Kaisha
US Patent
Chugai Seiyaku Kabushiki Kaisha
US Patent
Affinity DataIC50: 0.390nMAssay Description:Protein-protein interaction inhibition (PPI) between Kras and SOS1 was measured by energy transfer from nickel-conjugated donor beads to streptavidin...More data for this Ligand-Target Pair
Target InfoGoogleScholar
TargetGTPase KRas (G12D) and Son of Sevenless Homolog 1 (SOS)(Human)
Chugai Seiyaku Kabushiki Kaisha
US Patent
Chugai Seiyaku Kabushiki Kaisha
US Patent
Affinity DataIC50: 0.400nMAssay Description:Protein-protein interaction inhibition (PPI) between Kras and SOS1 was measured by energy transfer from nickel-conjugated donor beads to streptavidin...More data for this Ligand-Target Pair
Target InfoGoogleScholar
TargetGTPase KRas (G12D) and Son of Sevenless Homolog 1 (SOS)(Human)
Chugai Seiyaku Kabushiki Kaisha
US Patent
Chugai Seiyaku Kabushiki Kaisha
US Patent
Affinity DataIC50: 0.410nMAssay Description:Protein-protein interaction inhibition (PPI) between Kras and SOS1 was measured by energy transfer from nickel-conjugated donor beads to streptavidin...More data for this Ligand-Target Pair
Target InfoGoogleScholar
TargetGTPase KRas (G12D) and Son of Sevenless Homolog 1 (SOS)(Human)
Chugai Seiyaku Kabushiki Kaisha
US Patent
Chugai Seiyaku Kabushiki Kaisha
US Patent
Affinity DataIC50: 0.410nMAssay Description:Protein-protein interaction inhibition (PPI) between Kras and SOS1 was measured by energy transfer from nickel-conjugated donor beads to streptavidin...More data for this Ligand-Target Pair
Target InfoGoogleScholar
TargetGTPase KRas (G12D) and Son of Sevenless Homolog 1 (SOS)(Human)
Chugai Seiyaku Kabushiki Kaisha
US Patent
Chugai Seiyaku Kabushiki Kaisha
US Patent
Affinity DataIC50: 0.410nMAssay Description:Protein-protein interaction inhibition (PPI) between Kras and SOS1 was measured by energy transfer from nickel-conjugated donor beads to streptavidin...More data for this Ligand-Target Pair
Target InfoGoogleScholar
TargetGTPase KRas (G12D) and Son of Sevenless Homolog 1 (SOS)(Human)
Chugai Seiyaku Kabushiki Kaisha
US Patent
Chugai Seiyaku Kabushiki Kaisha
US Patent
Affinity DataIC50: 0.410nMAssay Description:Protein-protein interaction inhibition (PPI) between Kras and SOS1 was measured by energy transfer from nickel-conjugated donor beads to streptavidin...More data for this Ligand-Target Pair
Target InfoGoogleScholar
TargetGTPase KRas (G12D) and Son of Sevenless Homolog 1 (SOS)(Human)
Chugai Seiyaku Kabushiki Kaisha
US Patent
Chugai Seiyaku Kabushiki Kaisha
US Patent
Affinity DataIC50: 0.420nMAssay Description:Protein-protein interaction inhibition (PPI) between Kras and SOS1 was measured by energy transfer from nickel-conjugated donor beads to streptavidin...More data for this Ligand-Target Pair
Target InfoGoogleScholar


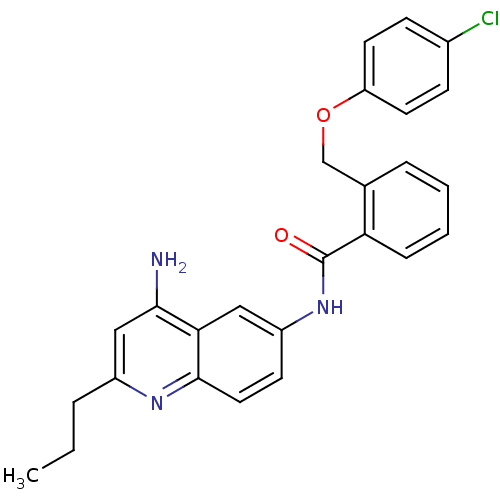
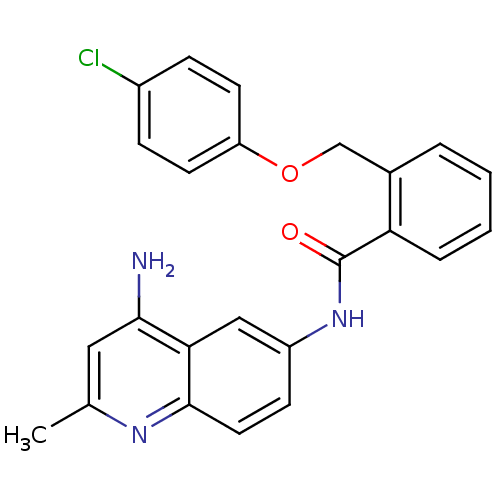
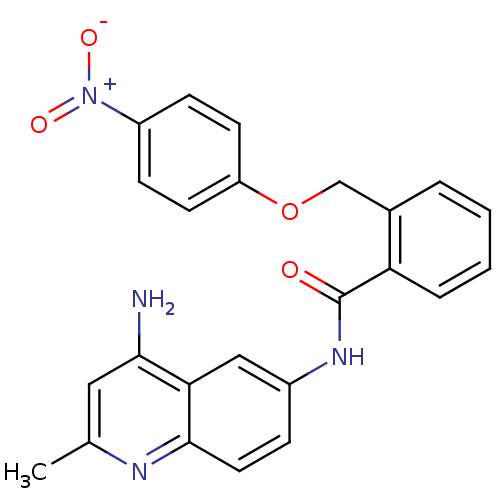
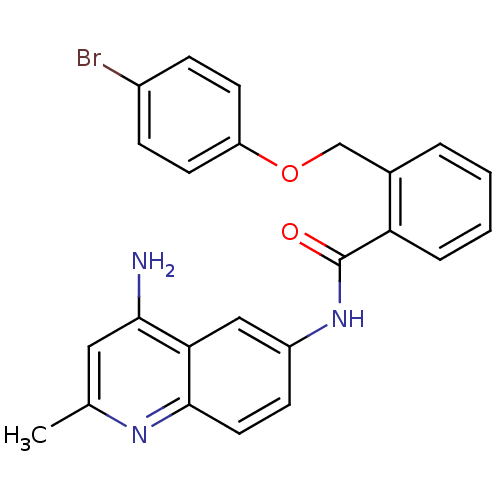

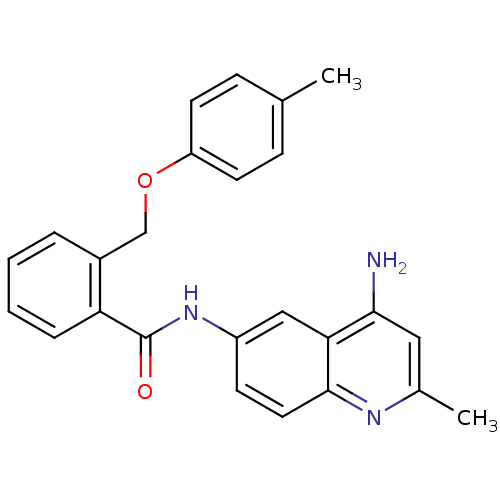
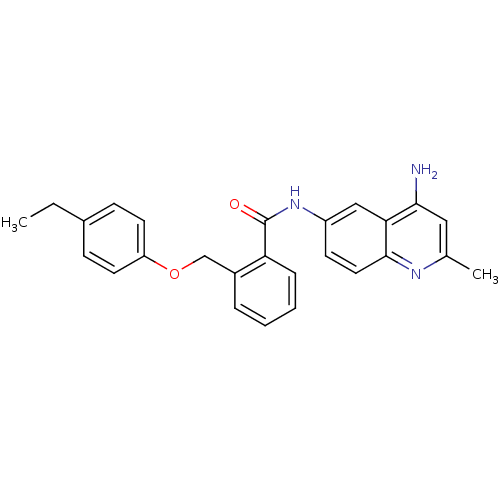

 3D Structure (crystal)
3D Structure (crystal)