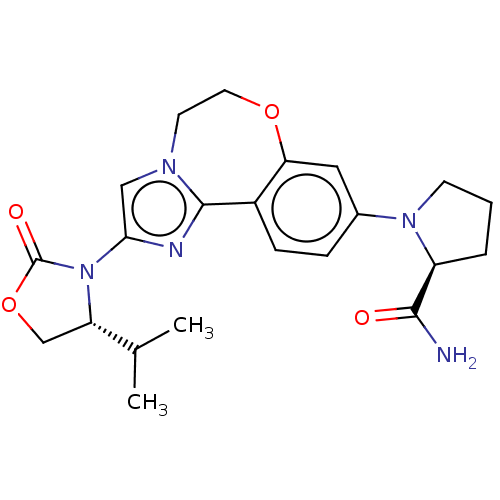
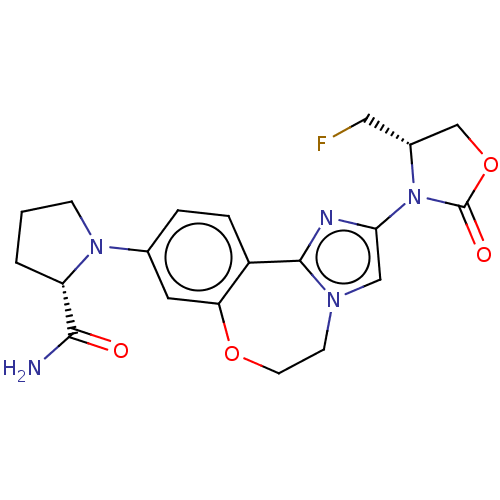
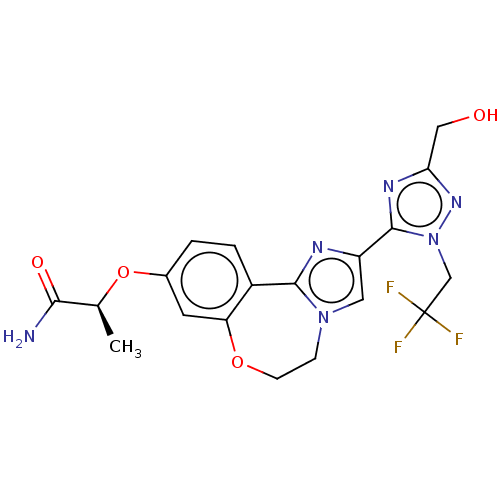
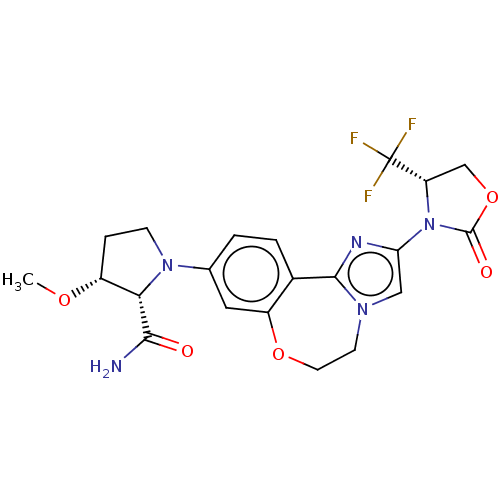
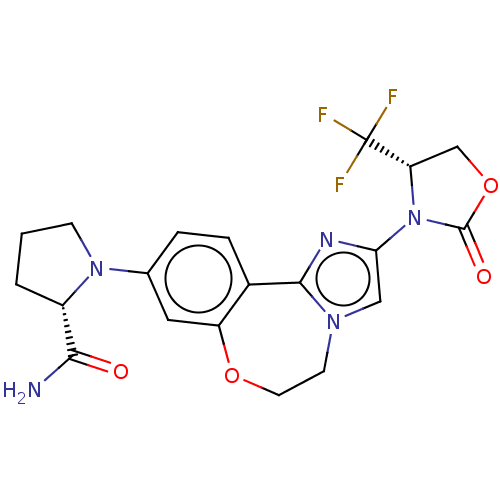
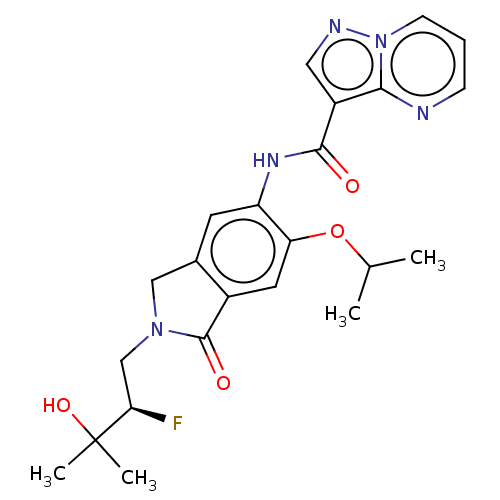
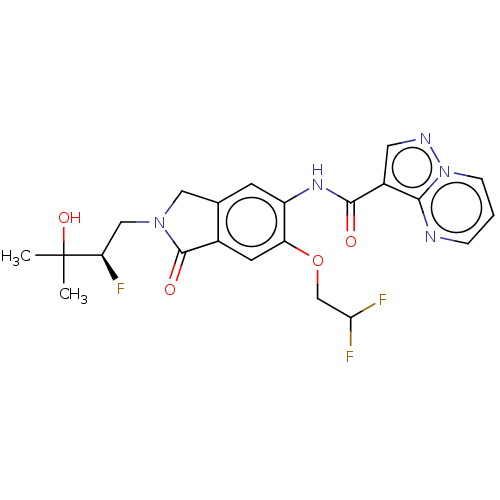
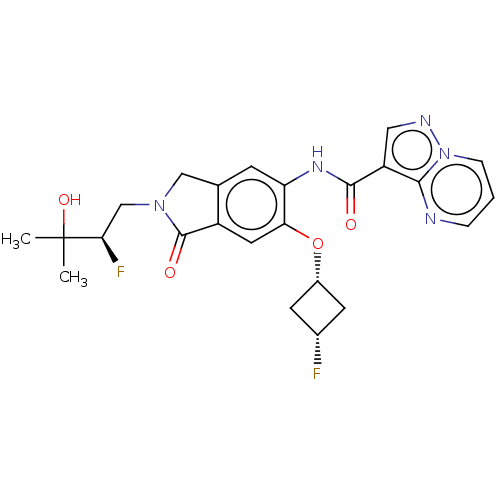
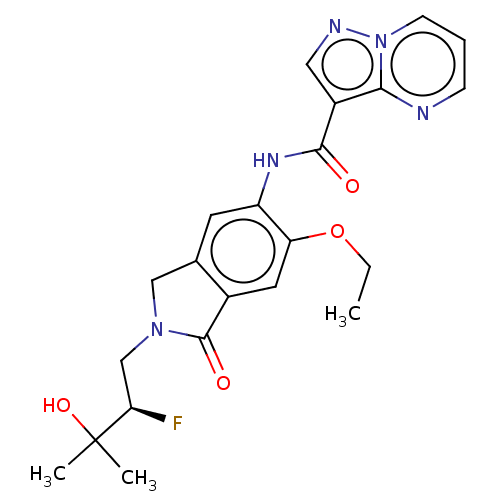
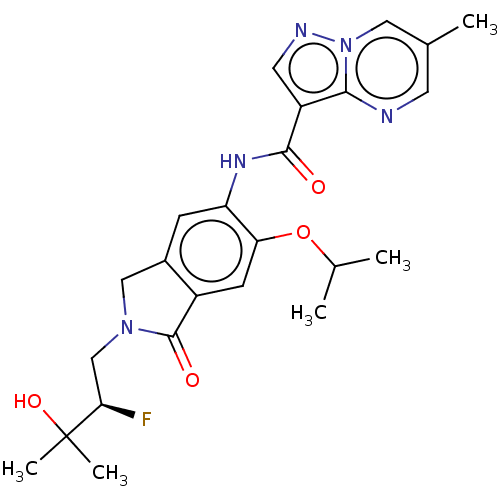
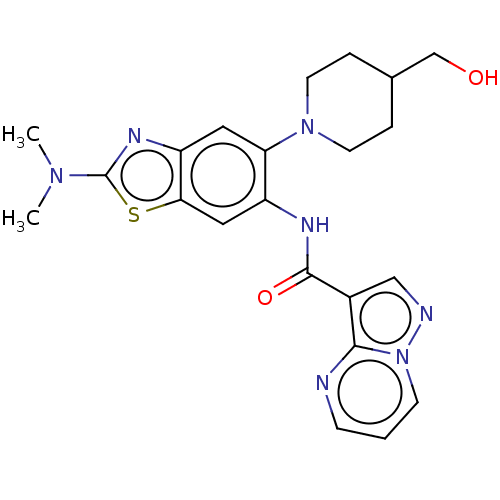
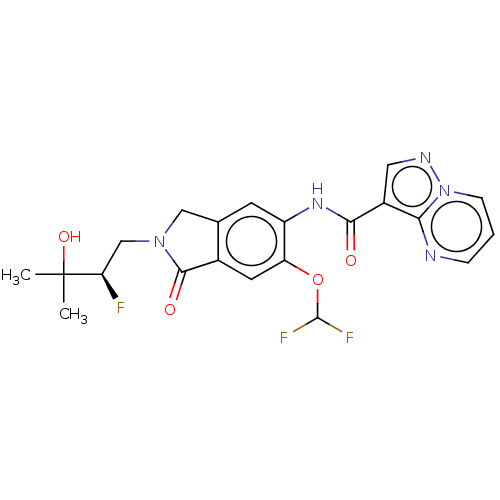
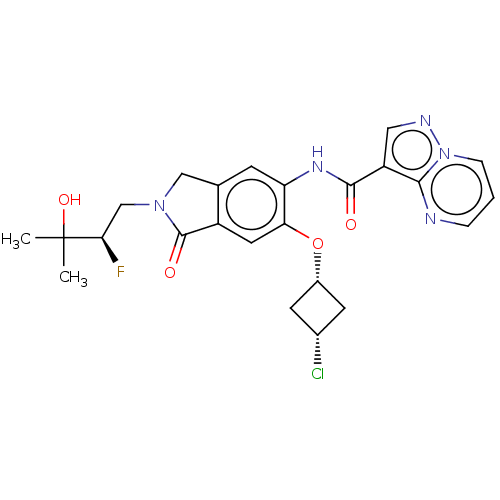
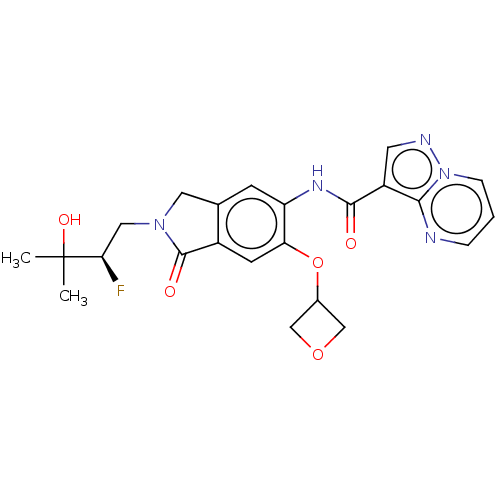
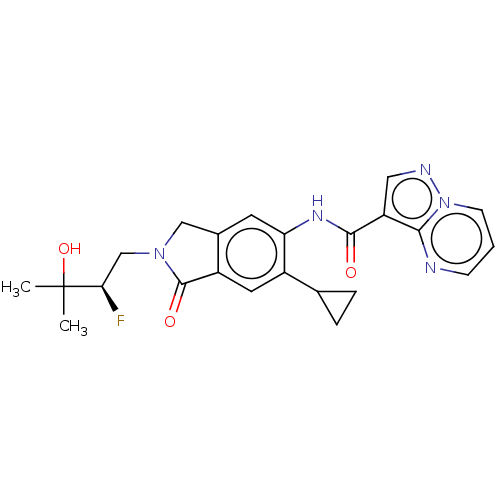
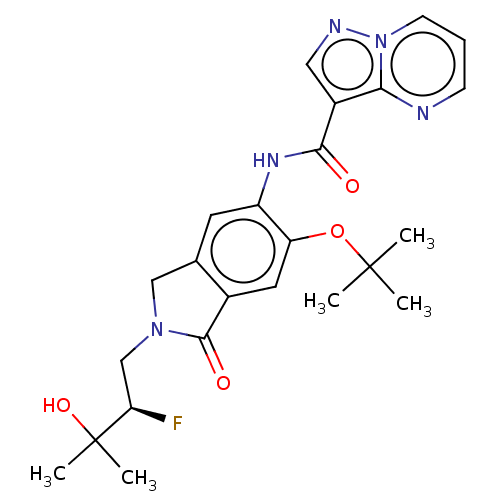
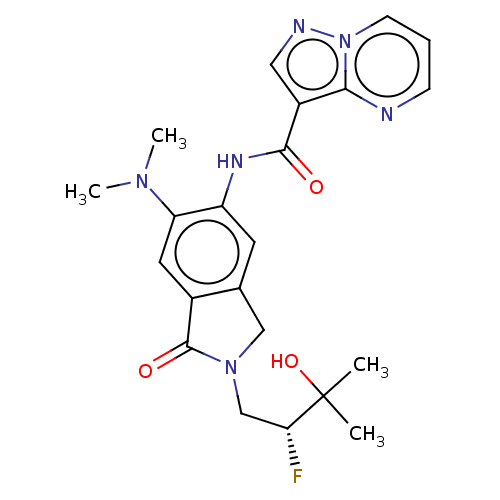
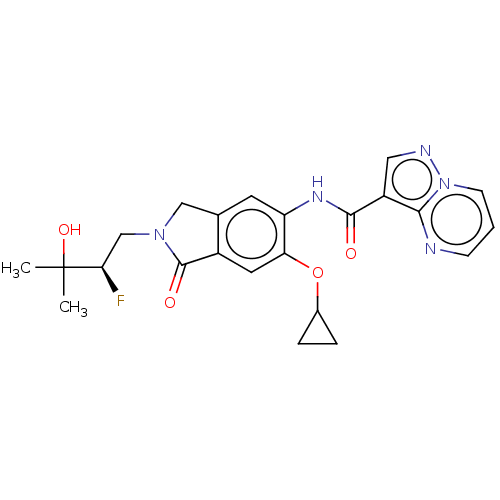
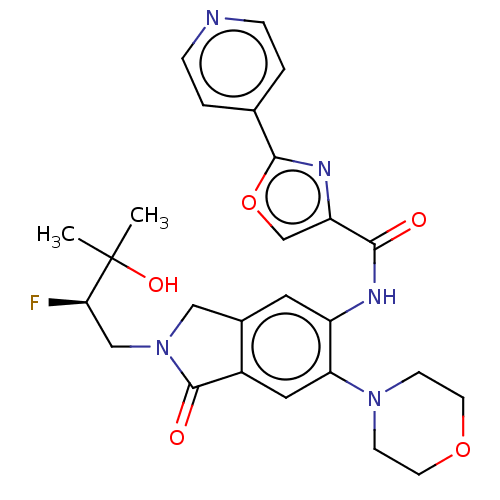
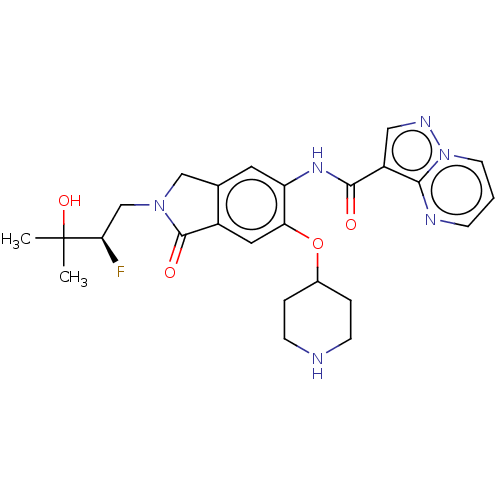
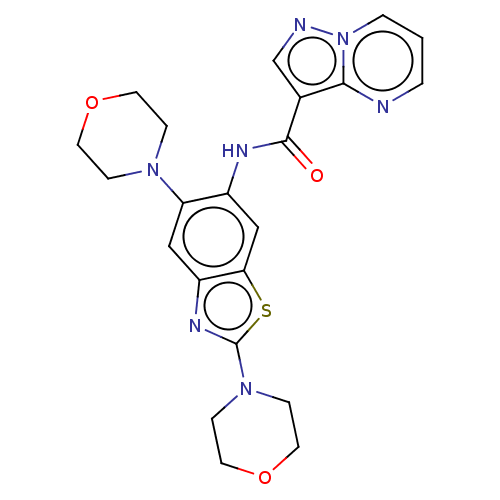
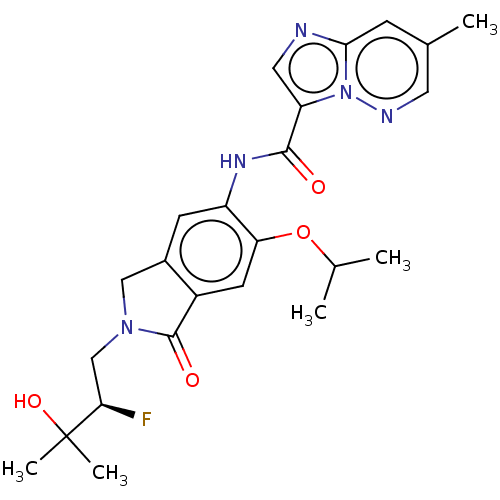
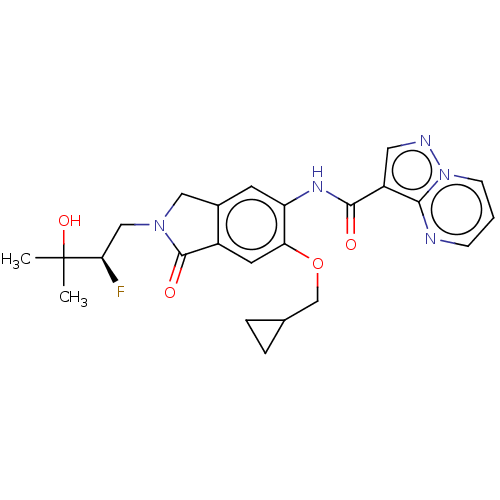
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.0260nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.0340nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.0420nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.0430nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.0510nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.0530nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.0600nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.0620nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.0900nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.0950nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.0970nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.100nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.107nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.150nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.157nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.170nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.188nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.260nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
Affinity DataKi: 0.280nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.289nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.320nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.323nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.346nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.355nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.370nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
Affinity DataKi: 0.420nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.440nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.490nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Inhibition of human recombinant full length His6-tagged IRAK4 expressed in baculovirus expression system using H-KKARFSRFAGSSPSQSSMVAR as substrate i...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Inhibition of human recombinant full length His6-tagged IRAK4 expressed in baculovirus expression system using H-KKARFSRFAGSSPSQSSMVAR as substrate i...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
Affinity DataKi: 0.510nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.560nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.590nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
Affinity DataKi: 0.610nMAssay Description:Kinase activities were assayed using the Transcreener-Fluorecescence polarization platform (BelBrook Labs, Madison, Wis., USA) that measures amounts ...More data for this Ligand-Target Pair
Affinity DataKi: 0.610nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
Affinity DataKi: 0.630nMAssay Description:Kinase activities were assayed using the Transcreener-Fluorecescence polarization platform (BelBrook Labs, Madison, Wis., USA) that measures amounts ...More data for this Ligand-Target Pair
Affinity DataKi: 0.630nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
Affinity DataKi: 0.630nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
Affinity DataKi: 0.670nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
Affinity DataKi: 0.680nMAssay Description:Kinase activities were assayed using the Transcreener-Fluorecescence polarization platform (BelBrook Labs, Madison, Wis., USA) that measures amounts ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.681nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.692nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
Affinity DataKi: 0.710nMAssay Description:The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.800nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:Inhibition of human recombinant full length His6-tagged IRAK4 expressed in baculovirus expression system using H-KKARFSRFAGSSPSQSSMVAR as substrate i...More data for this Ligand-Target Pair
Affinity DataKi: 0.820nMAssay Description:Kinase activities were assayed using the Transcreener-Fluorecescence polarization platform (BelBrook Labs, Madison, Wis., USA) that measures amounts ...More data for this Ligand-Target Pair
Affinity DataKi: 0.830nMAssay Description:Kinase activities were assayed using the Transcreener-Fluorecescence polarization platform (BelBrook Labs, Madison, Wis., USA) that measures amounts ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.853nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair


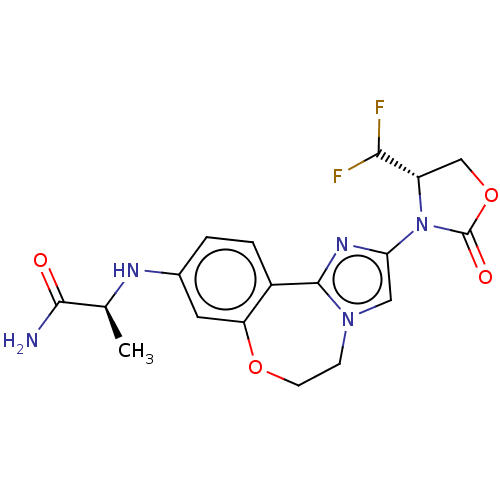
 3D Structure (crystal)
3D Structure (crystal)