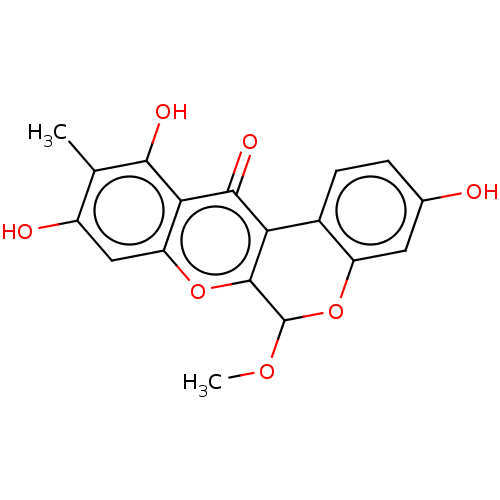
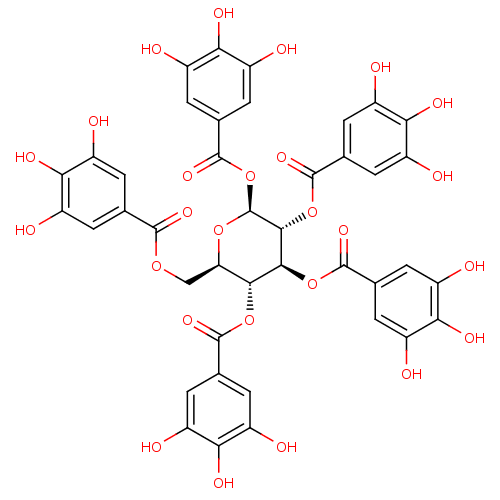
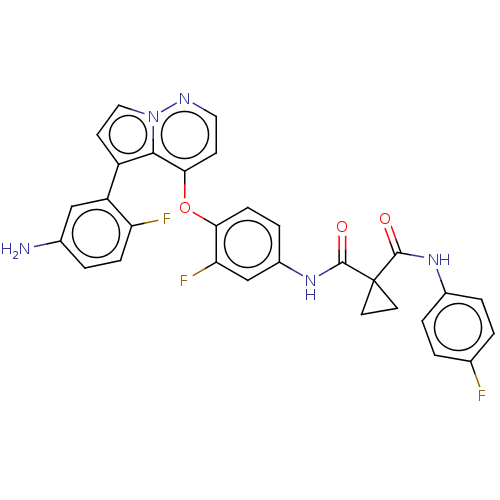
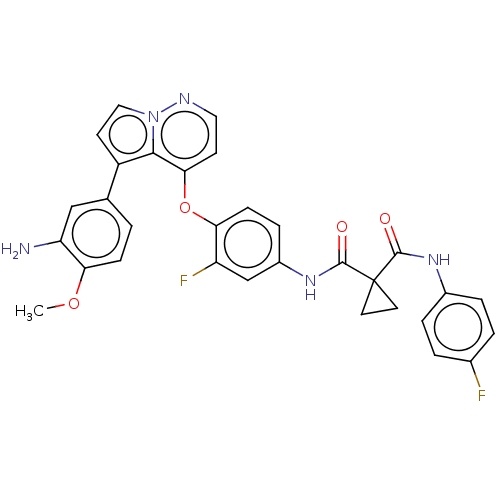
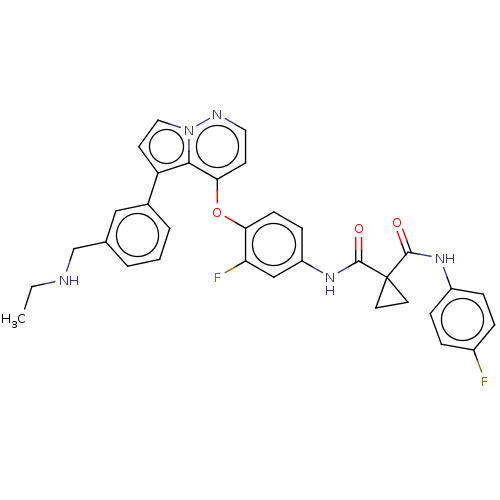
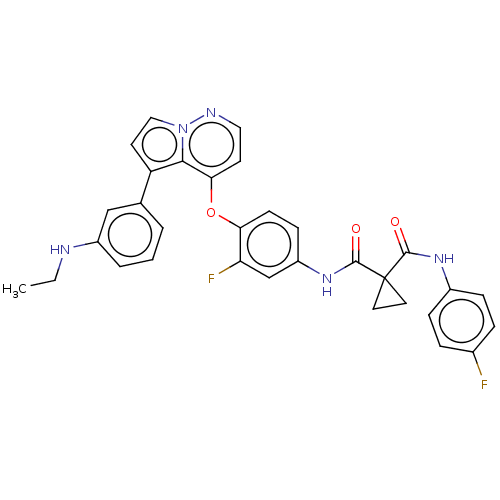
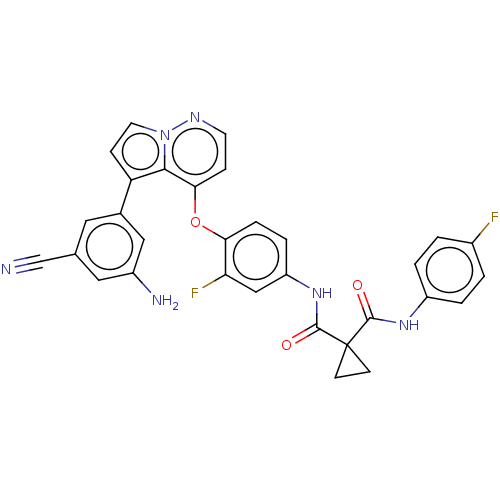
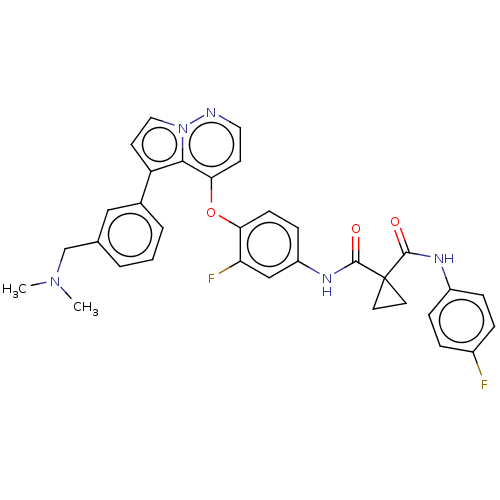
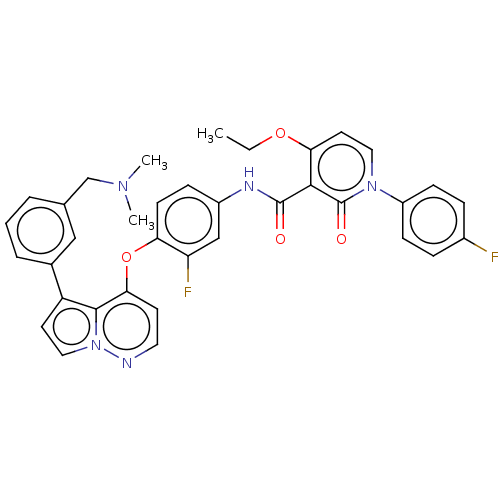
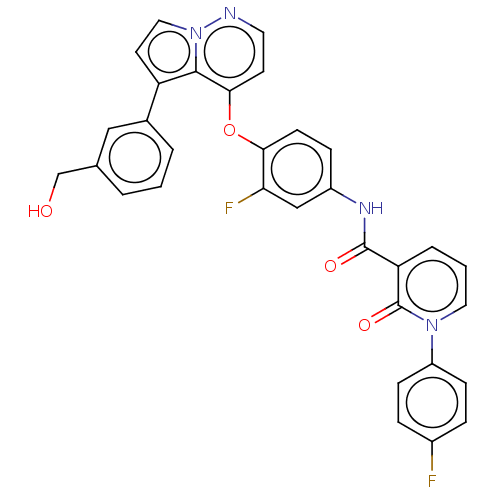
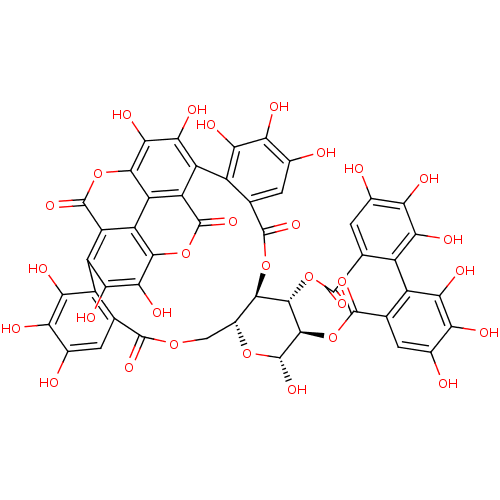
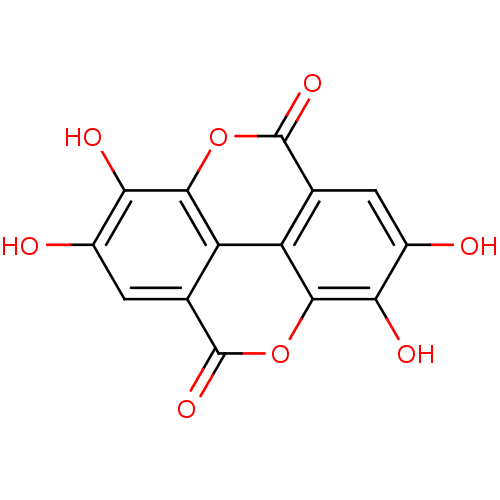
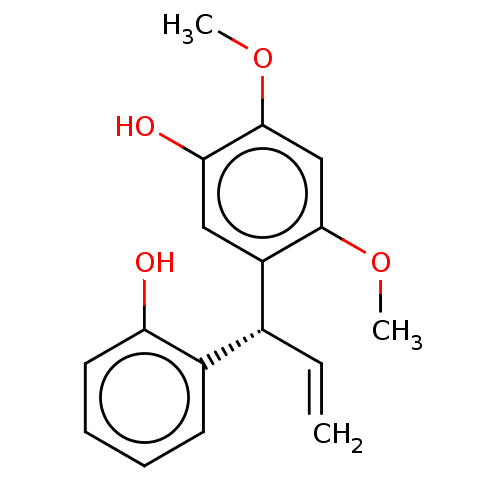
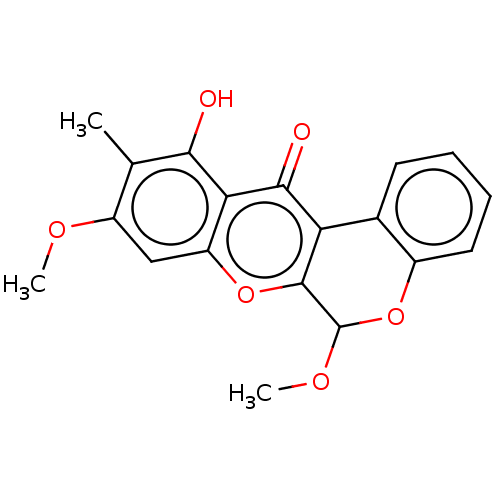
Affinity DataKi: 4.28E+3nMAssay Description:Non-competitive inhibition of BACE1 (unknown origin) by Dixon plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 5.01E+3nMAssay Description:Non-competitive inhibition of BACE1 (unknown origin) by Dixon plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 5.13E+3nMAssay Description:Inhibition of BACE1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 6.84E+3nMAssay Description:Inhibition of BACE1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: 410nMAssay Description:Inhibition of BACE1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.10E+3nMAssay Description:Inhibition of BACE1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.76E+3nMAssay Description:Inhibition of BACE1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.90E+3nMAssay Description:Inhibition of BACE1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 4.24E+3nMAssay Description:Inhibition of BACE1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 4.77E+3nMAssay Description:Inhibition of BACE1 (unknown origin)More data for this Ligand-Target Pair
TargetSterol O-acyltransferase 1(Rattus norvegicus)
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Affinity DataIC50: 6.00E+3nMAssay Description:In vitro inhibitory activity of compound was measured on rat liver Acyl coenzyme A:cholesterol acyltransferaseMore data for this Ligand-Target Pair
TargetSterol O-acyltransferase 1(Rattus norvegicus)
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:In vitro inhibitory activity of compound was measured on rat liver Acyl coenzyme A:cholesterol acyltransferaseMore data for this Ligand-Target Pair
TargetSterol O-acyltransferase 1(Rattus norvegicus)
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Affinity DataIC50: 1.20E+4nMAssay Description:In vitro inhibitory activity of compound was measured on rat liver Acyl coenzyme A:cholesterol acyltransferaseMore data for this Ligand-Target Pair
TargetSterol O-acyltransferase 1(Rattus norvegicus)
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Affinity DataIC50: 2.40E+4nMAssay Description:In vitro inhibitory activity of compound was measured on rat liver Acyl coenzyme A:cholesterol acyltransferaseMore data for this Ligand-Target Pair
TargetSterol O-acyltransferase 1(Rattus norvegicus)
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:In vitro inhibitory activity of compound was measured on rat liver Acyl coenzyme A:cholesterol acyltransferaseMore data for this Ligand-Target Pair
TargetSterol O-acyltransferase 1(Rattus norvegicus)
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Affinity DataIC50: 2.80E+4nMAssay Description:In vitro inhibitory activity of compound was measured on rat liver Acyl coenzyme A:cholesterol acyltransferaseMore data for this Ligand-Target Pair
TargetSterol O-acyltransferase 1(Rattus norvegicus)
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:In vitro inhibitory activity of compound was measured on rat liver Acyl coenzyme A:cholesterol acyltransferaseMore data for this Ligand-Target Pair
TargetSterol O-acyltransferase 1(Rattus norvegicus)
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Affinity DataIC50: 5.50E+4nMAssay Description:In vitro inhibitory activity of compound was measured on rat liver Acyl coenzyme A:cholesterol acyltransferaseMore data for this Ligand-Target Pair
Affinity DataIC50: 6.22E+4nMAssay Description:Inhibition of BACE1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+5nMAssay Description:Inhibition of BACE1 (unknown origin) expressed in CHO cells coexpressing human APP gene assessed as inhibition of beta-amyloid synthesisMore data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+6nMAssay Description:Inhibition of BACE1 (unknown origin)More data for this Ligand-Target Pair