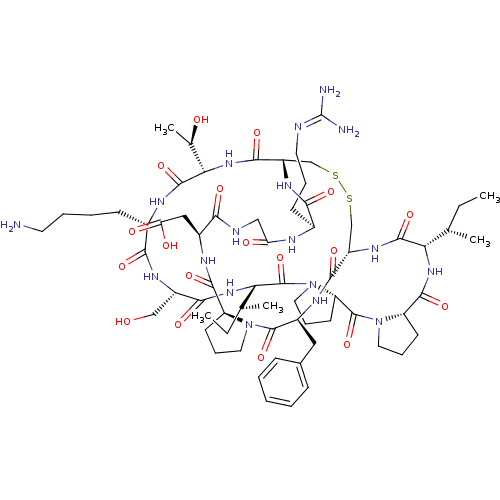
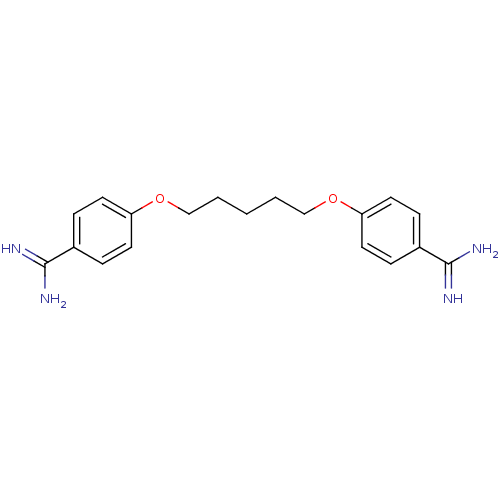
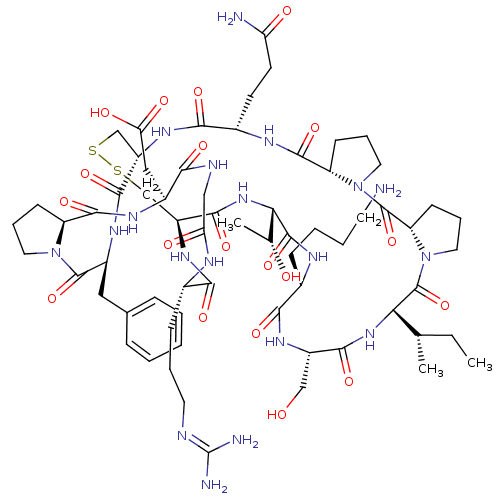
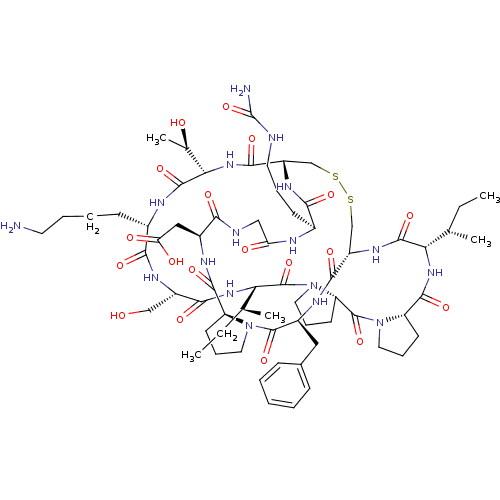
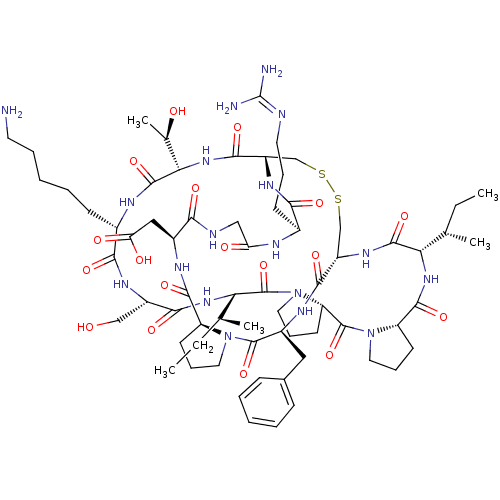
Affinity DataKi: 0.100nMAssay Description:Compound was tested for inhibition of Sunflower beta-trypsinMore data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 0.920nMAssay Description:Compound was tested for inhibition of Matriptase from human breast cancer cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Compound was tested for inhibition of bovine beta trypsinMore data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 100nM ΔG°: -39.6kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 160nM ΔG°: -38.4kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 191nMAssay Description:compound was tested for inhibitory activity against MatriptaseMore data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 208nMAssay Description:compound was tested for inhibitory activity against MatriptaseMore data for this Ligand-Target Pair
Affinity DataKi: 224nMAssay Description:compound was tested for inhibitory activity against ThrombinMore data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 380nM ΔG°: -36.3kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 450nM ΔG°: -35.9kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 535nMAssay Description:compound was tested for inhibitory activity against MatriptaseMore data for this Ligand-Target Pair
Affinity DataKi: 796nMAssay Description:compound was tested for inhibitory activity against ThrombinMore data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 857nM ΔG°: -34.3kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 860nM ΔG°: -34.3kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 890nM ΔG°: -34.2kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 924nMAssay Description:compound was tested for inhibitory activity against MatriptaseMore data for this Ligand-Target Pair
Affinity DataKi: 946nMAssay Description:compound was tested for inhibitory activity against ThrombinMore data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 1.00E+3nM ΔG°: -33.9kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 1.16E+3nMAssay Description:compound was tested for inhibitory activity against MatriptaseMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Georgetown University Medical Center
Curated by ChEMBL
Georgetown University Medical Center
Curated by ChEMBL
Affinity DataKi: 1.57E+3nMAssay Description:compound was tested for inhibitory activity against Urokinase-type plasminogen activator(microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Georgetown University Medical Center
Curated by ChEMBL
Georgetown University Medical Center
Curated by ChEMBL
Affinity DataKi: 1.95E+3nMAssay Description:compound was tested for inhibitory activity against Urokinase-type plasminogen activator(microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Georgetown University Medical Center
Curated by ChEMBL
Georgetown University Medical Center
Curated by ChEMBL
Affinity DataKi: 1.98E+3nMAssay Description:compound was tested for inhibitory activity against Urokinase-type plasminogen activator(microPa)More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 2.33E+3nM ΔG°: -31.8kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 2.67E+3nMAssay Description:compound was tested for inhibitory activity against ThrombinMore data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 4.50E+3nMAssay Description:compound was tested for inhibitory activity against MatriptaseMore data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 4.75E+3nM ΔG°: -30.1kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 5.00E+3nM ΔG°: -30.0kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 5.05E+3nMAssay Description:Compound was tested for inhibition of bovine ThrombinMore data for this Ligand-Target Pair
Affinity DataKi: 7.63E+3nM ΔG°: -28.9kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+4nM ΔG°: -28.3kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:compound was tested for inhibitory activity against MatriptaseMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Georgetown University Medical Center
Curated by ChEMBL
Georgetown University Medical Center
Curated by ChEMBL
Affinity DataKi: 1.44E+4nMAssay Description:compound was tested for inhibitory activity against Urokinase-type plasminogen activator(microPa)More data for this Ligand-Target Pair
Affinity DataKi: 1.63E+4nM ΔG°: -27.1kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 2.25E+4nM ΔG°: -26.3kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 2.56E+4nM ΔG°: -25.9kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 2.80E+4nM ΔG°: -25.7kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 3.36E+4nM ΔG°: -25.3kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 4.04E+4nM ΔG°: -24.8kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 4.56E+4nM ΔG°: -24.5kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 9.00E+4nM ΔG°: -22.9kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 1.07E+5nM ΔG°: -22.4kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 1.64E+5nM ΔG°: -21.4kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: >2.50E+5nM ΔG°: >-20.4kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
TargetUrokinase plasminogen activator surface receptor(Homo sapiens (Human))
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 5.00E+5nMAssay Description:Compound was tested for microPa) Urokinase-type plasminogen activator from human urineMore data for this Ligand-Target Pair
Affinity DataKi: 8.27E+5nM ΔG°: -17.4kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: >2.50E+6nM ΔG°: >-14.7kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Rattus norvegicus (rat))
The Hong Kong University Of Science And Technology
Curated by ChEMBL
The Hong Kong University Of Science And Technology
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Rattus norvegicus (rat))
The Hong Kong University Of Science And Technology
Curated by ChEMBL
The Hong Kong University Of Science And Technology
Curated by ChEMBL
Affinity DataIC50: 2.90nMAssay Description:Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Rattus norvegicus (rat))
The Hong Kong University Of Science And Technology
Curated by ChEMBL
The Hong Kong University Of Science And Technology
Curated by ChEMBL
Affinity DataIC50: 5.70nMAssay Description:Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate by Ellman's methodMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
National Tsing Hua University
Curated by ChEMBL
National Tsing Hua University
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of RET kinaseMore data for this Ligand-Target Pair