Affinity DataIC50: 0.0600nMAssay Description:Inhibition of PDE10A (unknown origin)More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 0.0600nMAssay Description:Inhibition of PDE10A (unknown origin)More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 0.110nMAssay Description:Inhibition of HDAC1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.120nMAssay Description:Inhibition of c-Met (unknown origin) using poly(Glu-Tyr) at 4:1 ratio as substrate in presence of ATP incubated for 60 mins by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 0.140nMAssay Description:Inhibition of PDE10A (unknown origin)More data for this Ligand-Target Pair
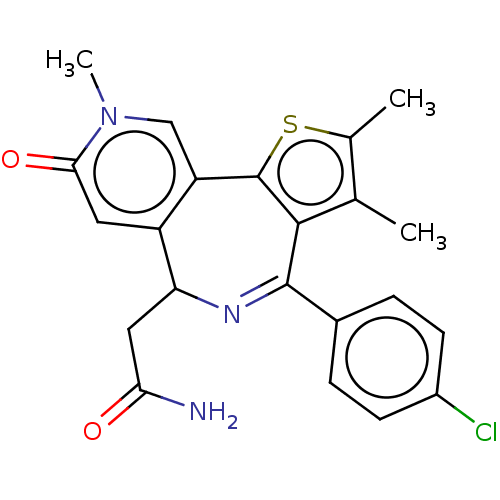
Ligand Info
Affinity DataIC50: 0.160nMAssay Description:Inhibition of PDE5 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.220nMAssay Description:Inhibition of PDE10A (unknown origin)More data for this Ligand-Target Pair
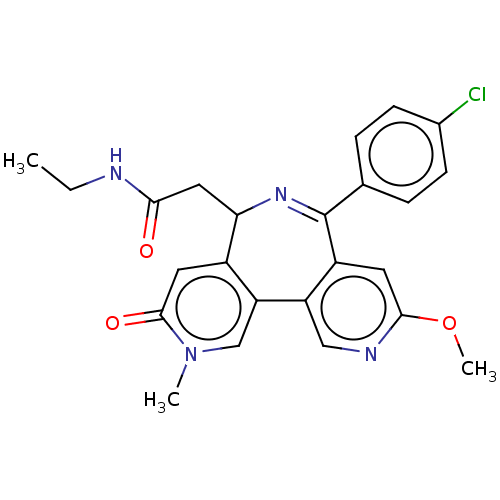
Ligand Info
Affinity DataIC50: 0.330nMAssay Description:Inhibition of HDAC2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.370nMAssay Description:Inhibition of HDAC11 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.460nMAssay Description:Inhibition of HDAC10 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: <0.5nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of PDE10A (unknown origin)More data for this Ligand-Target Pair
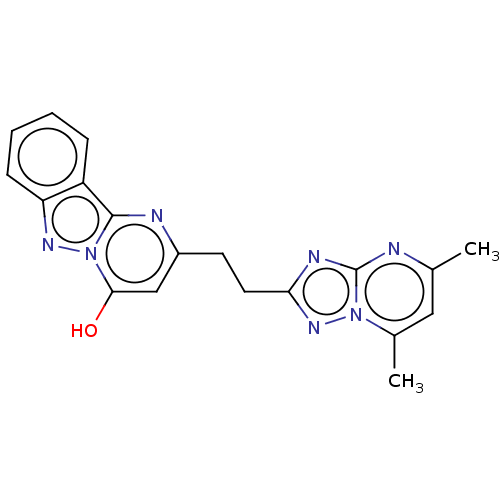
Ligand Info
Affinity DataIC50: <0.5nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 0.640nMAssay Description:Inhibition of HDAC4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.770nMAssay Description:Inhibition of PDE10A (unknown origin) by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibition of c-Met (unknown origin) kinase activity using poly(Glu-Tyr) at 4:1 ratio as substrate in presence of ATP incubated for 30 mins by HTRF a...More data for this Ligand-Target Pair
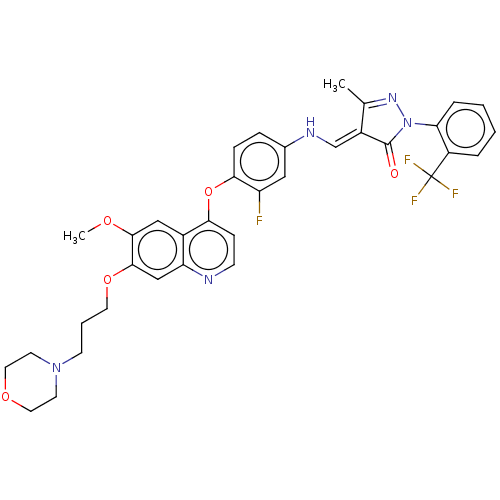
Ligand Info
Affinity DataIC50: 1.70nMAssay Description:Inhibition of HDAC1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:Inhibition of PDE10A (unknown origin)More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 1.80nMAssay Description:Inhibition of HDAC3 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:Inhibition of PDE10A2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Inhibition of PDE10A (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human PDE10A (449 to 789 residues) expressed in Escherichia coli BL21(DE3) using cAMP as substrate incubated for 30 mins by HTRF analys...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 2.20nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 2.20nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 2.20nMAssay Description:Inhibition of c-Met (unknown origin) kinase activity using poly(Glu-Tyr) at 4:1 ratio as substrate in presence of ATP incubated for 30 mins by HTRF a...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 2.80nMAssay Description:Inhibition of HDAC10 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.70nMAssay Description:Inhibition of HDAC5 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 4.30nMAssay Description:Inhibition of HDAC8 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 4.30nMAssay Description:Inhibition of c-Met (unknown origin) kinase activity using poly(Glu-Tyr) at 4:1 ratio as substrate in presence of ATP by TR-FRET assayMore data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 4.90nMAssay Description:Inhibition of HDAC3 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of HDAC2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Compounds were diluted by step-down dilution method (final concentration of DMSO was 1%) and added to the wells of a 384 well opti plate at desired c...More data for this Ligand-Target Pair
Affinity DataIC50: 5.10nMAssay Description:Inhibition of PDE10A (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 5.40nMAssay Description:Inhibition of HDAC11 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of c-Met (unknown origin) using poly(Glu-Tyr) at 4:1 ratio as substrate in presence of ATP incubated for 60 mins by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 6.5nMAssay Description:Inhibition of PDE10A (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 8.60nMAssay Description:Inhibition of c-Met (unknown origin) using poly(Glu-Tyr) at 4:1 ratio as substrate in presence of ATP incubated for 60 mins by ELISAMore data for this Ligand-Target Pair
Ligand Info

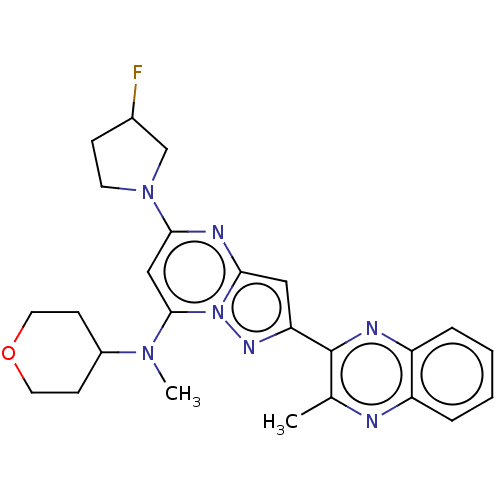

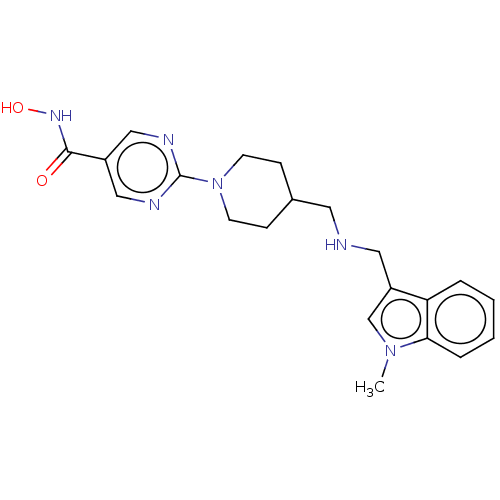


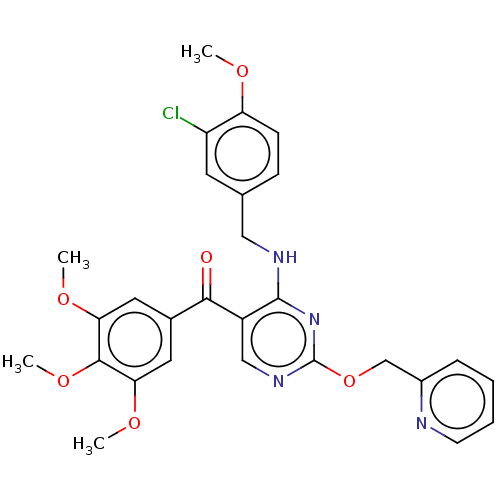
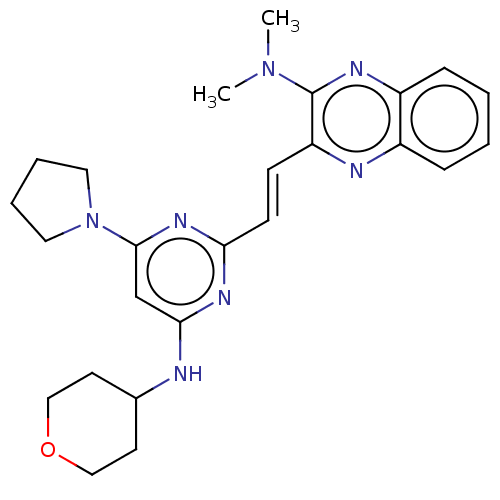
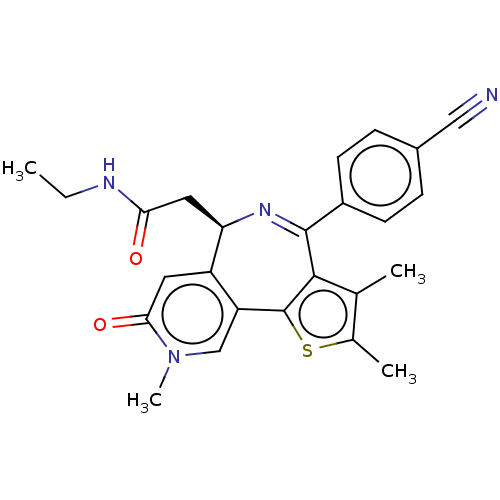

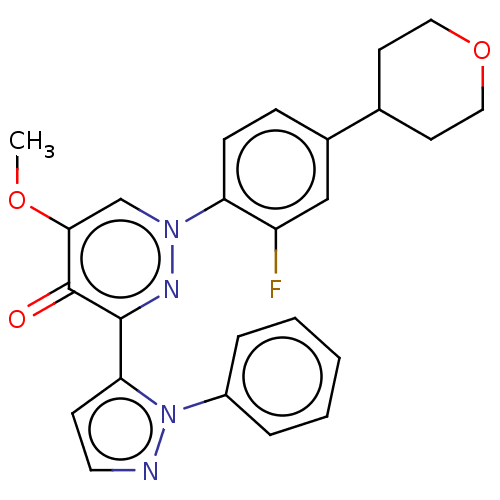


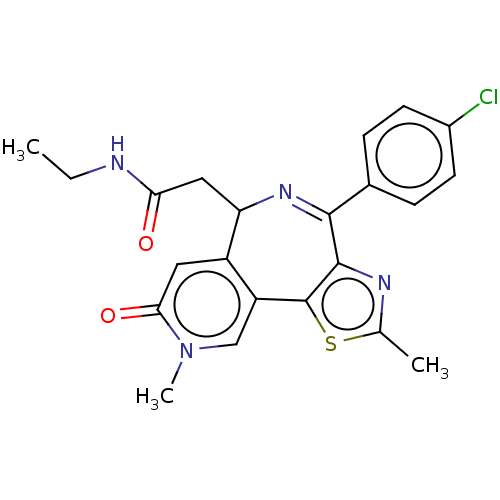




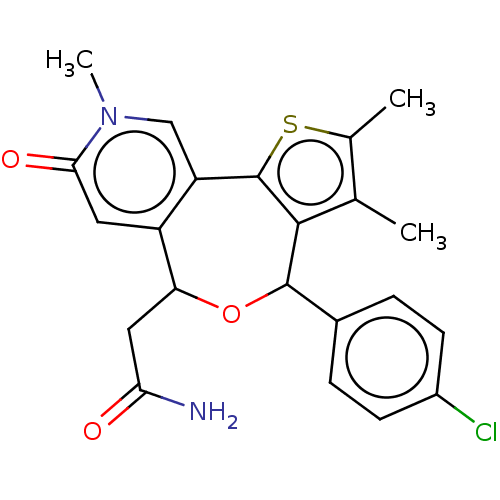

 3D Structure (crystal)
3D Structure (crystal)




