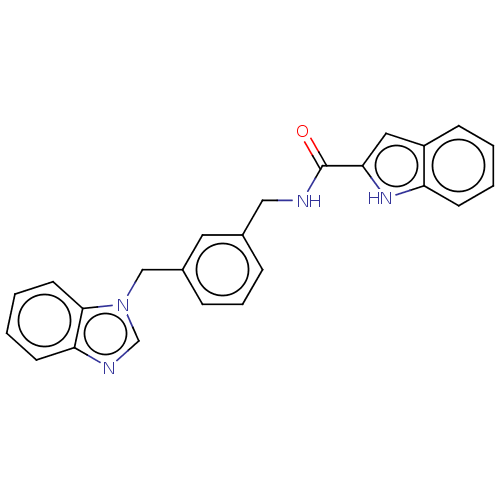
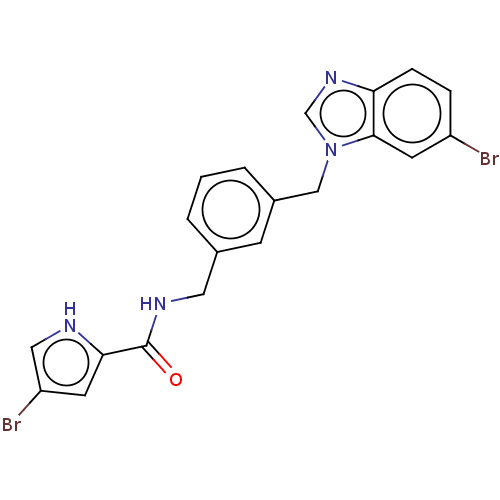
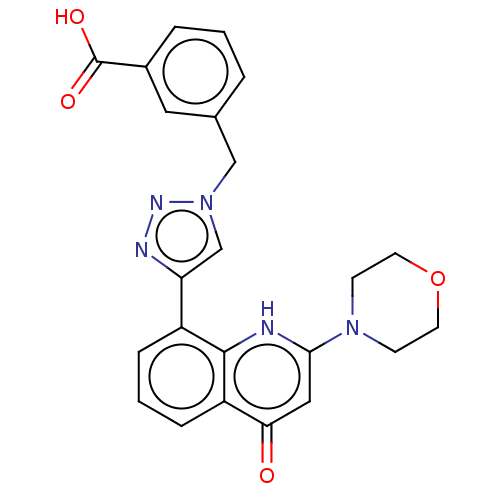
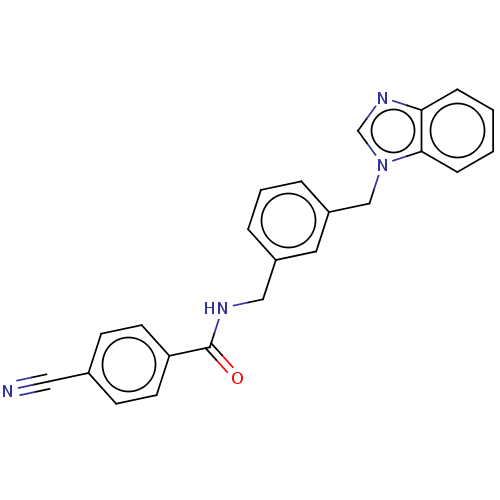
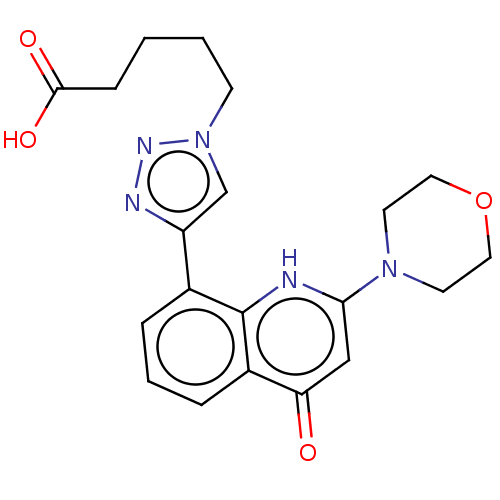
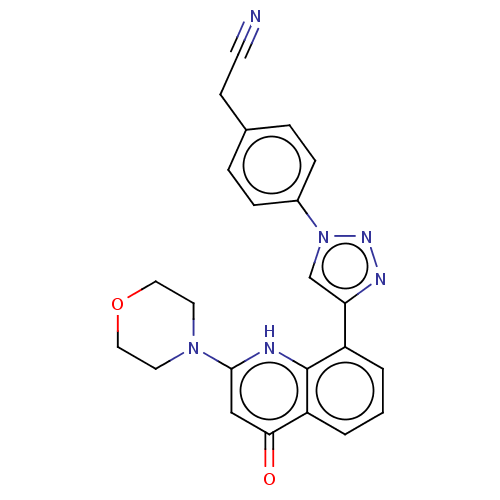
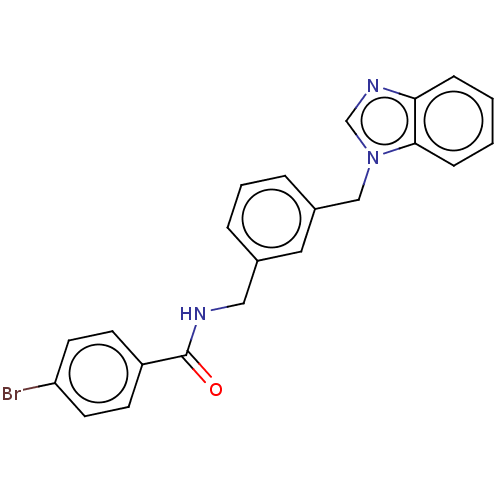
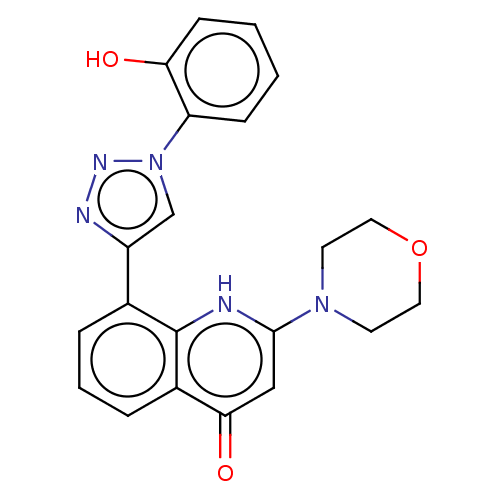
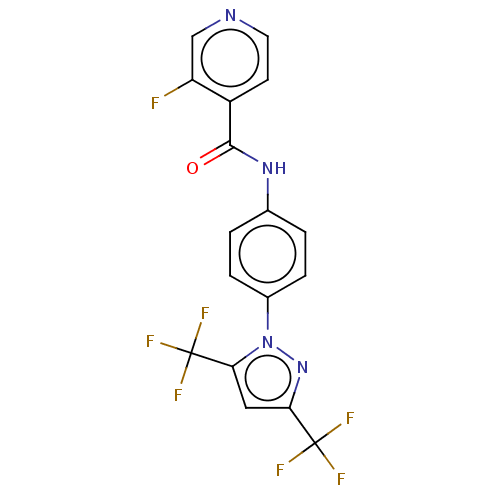
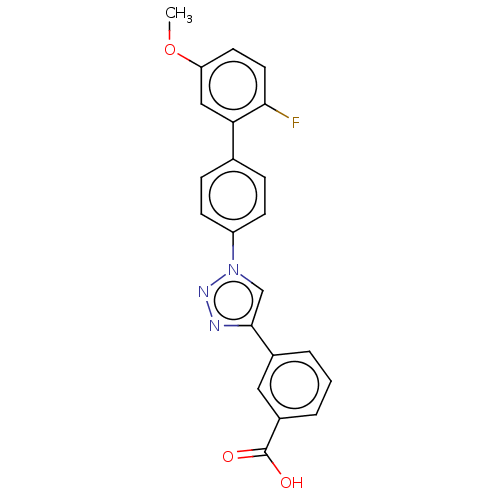
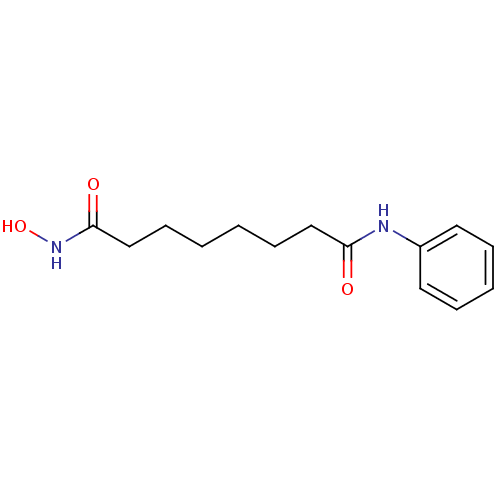
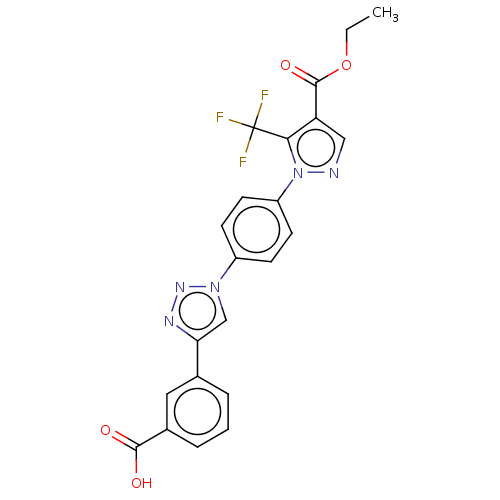
Affinity DataIC50: 7.70nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 9.30nMAssay Description:Inhibition of mouse IDO1 transfected in P815 cells assessed as reduction in L-Kyn level measured after 16 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human recombinant IDO1 assessed as reduction in kynurenine production using L-tryptophan incubated for 60 mins by Ehrlich's reagent bas...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Inhibition of mouse IDO1 transfected in P815 cells assessed as reduction in L-Kyn level measured after 16 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human LXF-289 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysisMore data for this Ligand-Target Pair
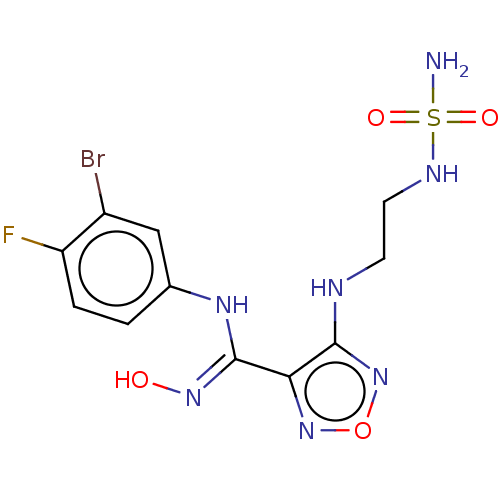
Affinity DataIC50: 16nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysisMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Universtà
US Patent
Universtà
US Patent
Affinity DataIC50: 20nMT: 2°CAssay Description:Some compounds of formula (I) described herein were assayed in vitro for their ability to inhibit the lipid kinase activity of PI3Ks, by using a non-...More data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human MCF7 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 43nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human HepG2 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 64nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 72nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 72nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:Inhibition of human recombinant IDO1 assessed as reduction in kynurenine production using L-tryptophan incubated for 60 mins by Ehrlich's reagent bas...More data for this Ligand-Target Pair
Affinity DataIC50: 83nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human MCF7 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 90nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysisMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataIC50: 90nMAssay Description:Antagonist activity at TRPV1 (unknown origin) expressed in human SH-SY5Y cells assessed as inhibition of capsaicin-induced calcium increase after 25 ...More data for this Ligand-Target Pair
Affinity DataIC50: 90nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysisMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Universtà
US Patent
Universtà
US Patent
Affinity DataIC50: 100nMT: 2°CAssay Description:Some compounds of formula (I) described herein were assayed in vitro for their ability to inhibit the lipid kinase activity of PI3Ks, by using a non-...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Universtà
US Patent
Universtà
US Patent
Affinity DataIC50: 100nMT: 2°CAssay Description:Some compounds of formula (I) described herein were assayed in vitro for their ability to inhibit the lipid kinase activity of PI3Ks, by using a non-...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Universtà
US Patent
Universtà
US Patent
Affinity DataIC50: 100nMT: 2°CAssay Description:Some compounds of formula (I) described herein were assayed in vitro for their ability to inhibit the lipid kinase activity of PI3Ks, by using a non-...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Universtà
US Patent
Universtà
US Patent
Affinity DataIC50: 100nMT: 2°CAssay Description:Some compounds of formula (I) described herein were assayed in vitro for their ability to inhibit the lipid kinase activity of PI3Ks, by using a non-...More data for this Ligand-Target Pair
Affinity DataIC50: 110nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human MCF7 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 112nMAssay Description:Inhibition of mouse IDO1 transfected in P815 cells assessed as reduction in L-Kyn level measured after 16 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 134nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 140nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human DAN-G cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysisMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Universtà
US Patent
Universtà
US Patent
Affinity DataIC50: 140nMT: 2°CAssay Description:Some compounds of formula (I) described herein were assayed in vitro for their ability to inhibit the lipid kinase activity of PI3Ks, by using a non-...More data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibition of human recombinant IDO1 assessed as reduction in kynurenine production using L-tryptophan incubated for 60 mins by Ehrlich's reagent bas...More data for this Ligand-Target Pair
TargetCalcium release-activated calcium channel protein/Stromal interaction molecule 1(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataIC50: 228nMAssay Description:Inhibition of STIM1/Orai1 (unknown origin) expressed in HEK293 cells assessed as reduction in tBHQ-induced calcium responseMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Universtà
US Patent
Universtà
US Patent
Affinity DataIC50: 260nMT: 2°CAssay Description:Some compounds of formula (I) described herein were assayed in vitro for their ability to inhibit the lipid kinase activity of PI3Ks, by using a non-...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Universtà
US Patent
Universtà
US Patent
Affinity DataIC50: 260nMT: 2°CAssay Description:Some compounds of formula (I) described herein were assayed in vitro for their ability to inhibit the lipid kinase activity of PI3Ks, by using a non-...More data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Modulation of TRPC1/TRPC3/STIM1/Orai1 in HEK cells assessed as induction of store-operated calcium entry by measuring residual activity preincubated ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Universtà
US Patent
Universtà
US Patent
Affinity DataIC50: 320nMT: 2°CAssay Description:Some compounds of formula (I) described herein were assayed in vitro for their ability to inhibit the lipid kinase activity of PI3Ks, by using a non-...More data for this Ligand-Target Pair
Affinity DataIC50: 327nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 340nMAssay Description:Inhibition of human recombinant IDO1 assessed as reduction in kynurenine production using L-tryptophan incubated for 60 mins by Ehrlich's reagent bas...More data for this Ligand-Target Pair
TargetCalcium release-activated calcium channel protein/Stromal interaction molecule 1(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataIC50: 361nMAssay Description:Inhibition of STIM1/Orai1 (unknown origin) expressed in HEK293 cells assessed as reduction in tBHQ-induced calcium responseMore data for this Ligand-Target Pair
Affinity DataIC50: 407nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 413nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 450nMAssay Description:Inhibition of human recombinant IDO1 assessed as reduction in kynurenine production using L-tryptophan incubated for 60 mins by Ehrlich's reagent bas...More data for this Ligand-Target Pair
Affinity DataIC50: 477nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysisMore data for this Ligand-Target Pair
TargetHistone deacetylase(Homo sapiens (Human))
Universita Degli Studi Del Piemonte Orientale A. Avogadro
Curated by ChEMBL
Universita Degli Studi Del Piemonte Orientale A. Avogadro
Curated by ChEMBL
Affinity DataIC50: 500nMAssay Description:Inhibition of HDAC in human SHSY5Y cells by fluorimetric cellular activity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 500nMAssay Description:Modulation of TRPC1/TRPC3/STIM1/Orai1 in HEK cells assessed as induction of store-operated calcium entry by measuring residual activity preincubated ...More data for this Ligand-Target Pair
Affinity DataIC50: 580nMAssay Description:Inhibition of human recombinant IDO1 assessed as reduction in kynurenine production using L-tryptophan incubated for 60 mins by Ehrlich's reagent bas...More data for this Ligand-Target Pair
TargetCalcium release-activated calcium channel protein 1/Short transient receptor potential channel 1/Short transient receptor potential channel 3/Stromal interaction molecule 1(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataIC50: 600nMAssay Description:Modulation of TRPC1/TRPC3/STIM1/Orai1 in HEK cells assessed as induction of store-operated calcium entry by measuring residual activity preincubated ...More data for this Ligand-Target Pair
Affinity DataIC50: 605nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human DAN-G cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 630nMAssay Description:Inhibition of human recombinant IDO1 assessed as reduction in kynurenine production using L-tryptophan incubated for 60 mins by Ehrlich's reagent bas...More data for this Ligand-Target Pair
Affinity DataIC50: 636nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 690nMAssay Description:Inhibition of human recombinant IDO1 assessed as reduction in kynurenine production using L-tryptophan incubated for 60 mins by Ehrlich's reagent bas...More data for this Ligand-Target Pair
Affinity DataIC50: 730nMAssay Description:Inhibition of human recombinant IDO1 assessed as reduction in kynurenine production using L-tryptophan incubated for 60 mins by Ehrlich's reagent bas...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Universtà
US Patent
Universtà
US Patent
Affinity DataIC50: 730nMT: 2°CAssay Description:Some compounds of formula (I) described herein were assayed in vitro for their ability to inhibit the lipid kinase activity of PI3Ks, by using a non-...More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)