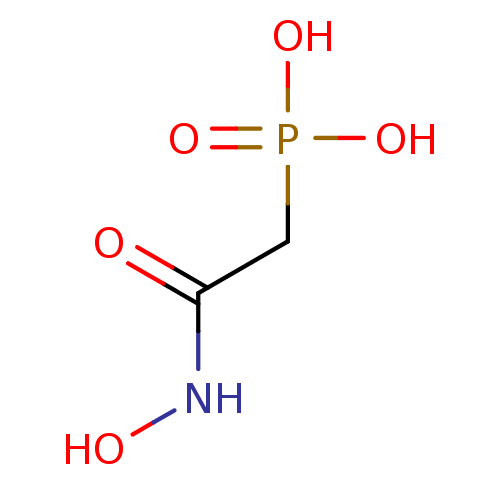
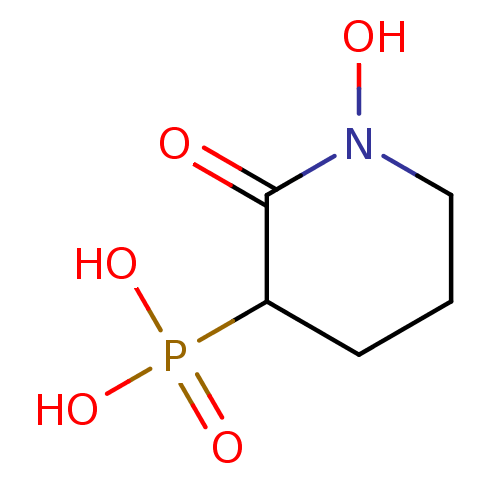
Affinity DataIC50: 0.0300nMAssay Description:Inhibition of EGFRMore data for this Ligand-Target Pair
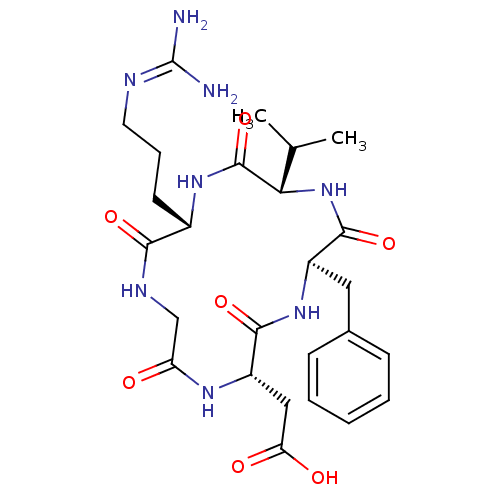
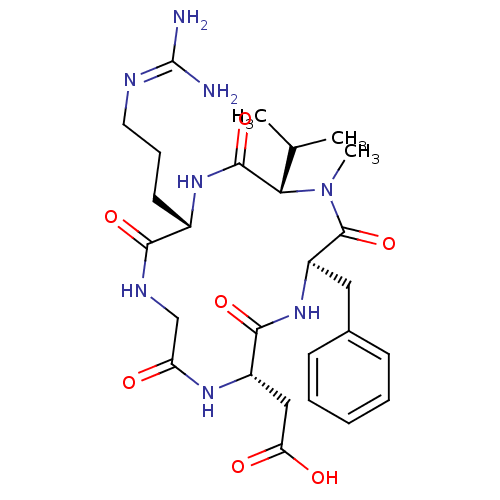
TargetIntegrin alpha-V/beta-5(Homo sapiens (Human))
Department Of Organic Chemistry Ugo Schiff University Of Firenze Via Della Lastruccia 13
Curated by ChEMBL
Department Of Organic Chemistry Ugo Schiff University Of Firenze Via Della Lastruccia 13
Curated by ChEMBL
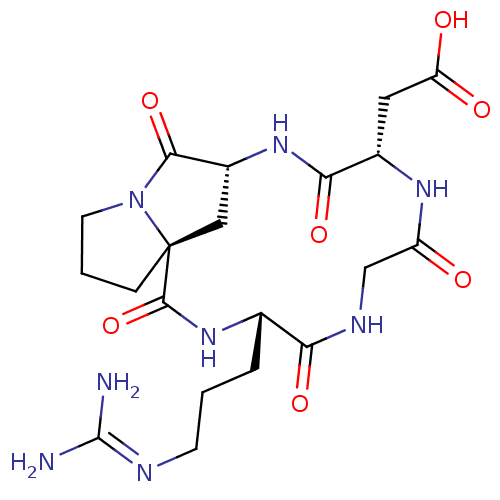
Affinity DataIC50: 0.110nMAssay Description:Displacement of [125I]Echistatin from human placental integrin alpha-V-beta-5 receptorMore data for this Ligand-Target Pair
TargetIntegrin alpha-V/beta-5(Homo sapiens (Human))
Department Of Organic Chemistry Ugo Schiff University Of Firenze Via Della Lastruccia 13
Curated by ChEMBL
Department Of Organic Chemistry Ugo Schiff University Of Firenze Via Della Lastruccia 13
Curated by ChEMBL
Affinity DataIC50: 0.130nMAssay Description:Displacement of [125I]Echistatin from human placental integrin alpha-V-beta-5 receptorMore data for this Ligand-Target Pair
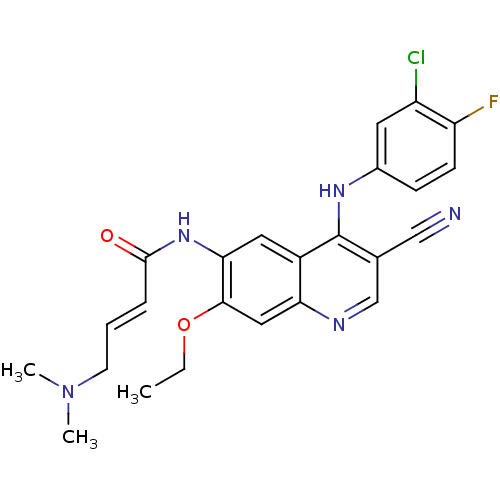
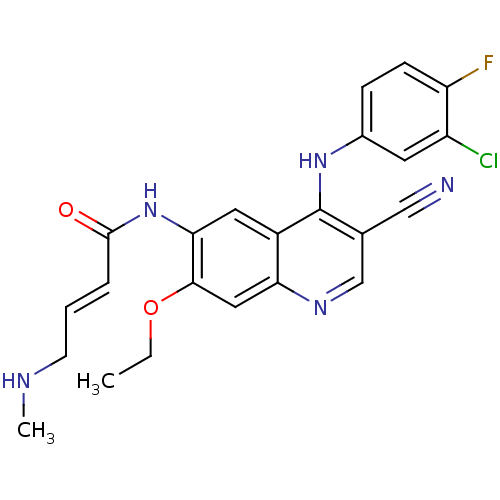
TargetAlpha-enolase(Homo sapiens (Human))
University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of ENO1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 5.35nMAssay Description:Inhibition of EGFR autophosphorylation in human A431 cellsMore data for this Ligand-Target Pair
TargetAlpha-enolase(Homo sapiens (Human))
University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 6.30nMAssay Description:Inhibition of ENO1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 8.02nMAssay Description:Inhibition of EGFR autophosphorylation in human A431 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 8.12nMAssay Description:Inhibition of EGFR autophosphorylation in human A431 cellsMore data for this Ligand-Target Pair
TargetIntegrin alpha-V/beta-5(Homo sapiens (Human))
Department Of Organic Chemistry Ugo Schiff University Of Firenze Via Della Lastruccia 13
Curated by ChEMBL
Department Of Organic Chemistry Ugo Schiff University Of Firenze Via Della Lastruccia 13
Curated by ChEMBL
Affinity DataIC50: 14.3nMAssay Description:Displacement of [125I]Echistatin from human placental integrin alpha-V-beta-5 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 16.5nMAssay Description:Inhibition of EGFR autophosphorylation in human A431 cellsMore data for this Ligand-Target Pair
TargetIntegrin alpha-V/beta-3(Homo sapiens (Human))
Department Of Organic Chemistry Ugo Schiff University Of Firenze Via Della Lastruccia 13
Curated by ChEMBL
Department Of Organic Chemistry Ugo Schiff University Of Firenze Via Della Lastruccia 13
Curated by ChEMBL
Affinity DataIC50: 18.9nMAssay Description:Displacement of [125I]echistatin from human placental integrin alphaVbeta3 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 22.0nMAssay Description:Inhibition of EGFR autophosphorylation in human A431 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 23.0nMAssay Description:Inhibition of EGFR autophosphorylation in human A431 cellsMore data for this Ligand-Target Pair
TargetIntegrin alpha-V/beta-3(Homo sapiens (Human))
Department Of Organic Chemistry Ugo Schiff University Of Firenze Via Della Lastruccia 13
Curated by ChEMBL
Department Of Organic Chemistry Ugo Schiff University Of Firenze Via Della Lastruccia 13
Curated by ChEMBL
Affinity DataIC50: 34.4nMAssay Description:Displacement of [125I]echistatin from human placental integrin alphaVbeta3 receptorMore data for this Ligand-Target Pair
TargetAlpha-enolase(Homo sapiens (Human))
University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 37.9nMpH: 7.4Assay Description:Enolase activity was measured by two different methods: a fluorometric NADH-linked assay or adirect spectrophotometric assay via formation of PEP. In...More data for this Ligand-Target Pair
Affinity DataIC50: 42.5nMpH: 7.4Assay Description:Enolase activity was measured by two different methods: a fluorometric NADH-linked assay or adirect spectrophotometric assay via formation of PEP. In...More data for this Ligand-Target Pair
TargetAlpha-enolase(Homo sapiens (Human))
University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 51nMAssay Description:Inhibition of ENO1 (unknown origin)More data for this Ligand-Target Pair
TargetAlpha-enolase(Homo sapiens (Human))
University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 53.2nMpH: 7.4Assay Description:Enolase activity was measured by two different methods: a fluorometric NADH-linked assay or adirect spectrophotometric assay via formation of PEP. In...More data for this Ligand-Target Pair
Affinity DataIC50: 60.2nMAssay Description:Inhibition of EGFR autophosphorylation in human A431 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 62.3nMpH: 7.4Assay Description:Enolase activity was measured by two different methods: a fluorometric NADH-linked assay or adirect spectrophotometric assay via formation of PEP. In...More data for this Ligand-Target Pair
TargetIntegrin alpha-V/beta-3(Homo sapiens (Human))
Department Of Organic Chemistry Ugo Schiff University Of Firenze Via Della Lastruccia 13
Curated by ChEMBL
Department Of Organic Chemistry Ugo Schiff University Of Firenze Via Della Lastruccia 13
Curated by ChEMBL
Affinity DataIC50: 196nMAssay Description:Displacement of [125I]echistatin from human placental integrin alphaVbeta3 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+3nMAssay Description:Inhibition of EGFR autophosphorylation in human A431 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+3nMAssay Description:Inhibition of EGFR autophosphorylation in human A431 cellsMore data for this Ligand-Target Pair
TargetIntegrin alpha-V/beta-3(Homo sapiens (Human))
Department Of Organic Chemistry Ugo Schiff University Of Firenze Via Della Lastruccia 13
Curated by ChEMBL
Department Of Organic Chemistry Ugo Schiff University Of Firenze Via Della Lastruccia 13
Curated by ChEMBL
Affinity DataIC50: 2.15E+3nMAssay Description:Displacement of [125I]echistatin from human placental integrin alphaVbeta3 receptorMore data for this Ligand-Target Pair
TargetAlpha-enolase(Homo sapiens (Human))
University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 2.69E+3nMAssay Description:Inhibition of ENO1 (unknown origin)More data for this Ligand-Target Pair
TargetAlpha-enolase(Homo sapiens (Human))
University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 5.90E+3nMAssay Description:Inhibition of ENO1 (unknown origin)More data for this Ligand-Target Pair
TargetIntegrin alpha-V/beta-5(Homo sapiens (Human))
Department Of Organic Chemistry Ugo Schiff University Of Firenze Via Della Lastruccia 13
Curated by ChEMBL
Department Of Organic Chemistry Ugo Schiff University Of Firenze Via Della Lastruccia 13
Curated by ChEMBL
Affinity DataIC50: 7.96E+3nMAssay Description:Displacement of [125I]Echistatin from human placental integrin alpha-V-beta-5 receptorMore data for this Ligand-Target Pair

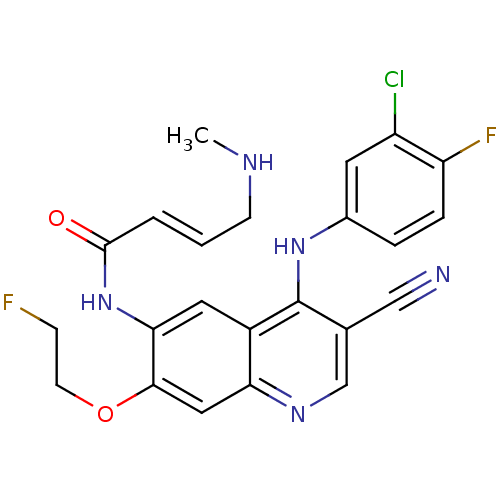
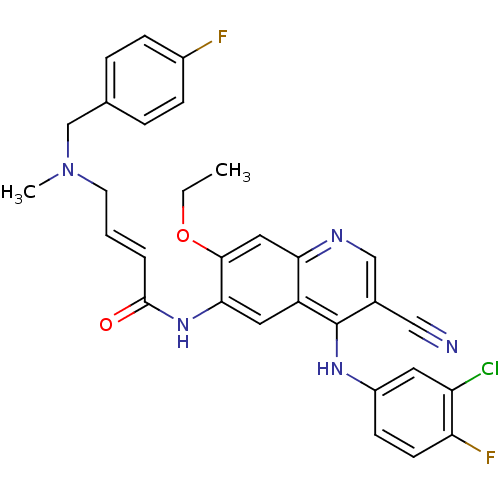
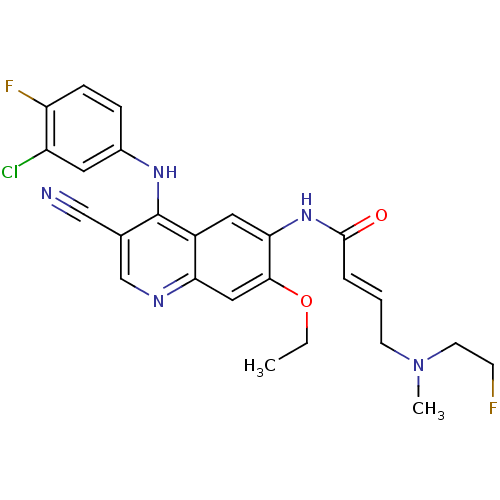





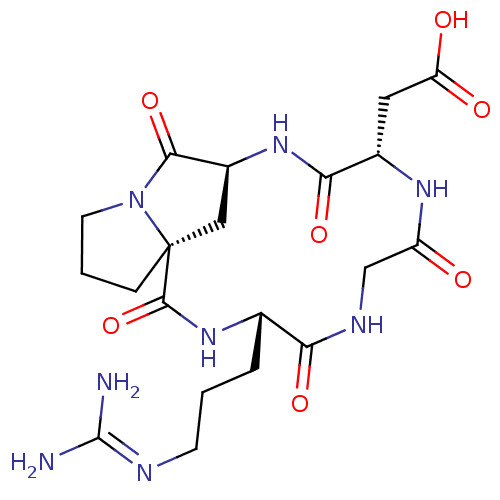

 3D Structure (crystal)
3D Structure (crystal)