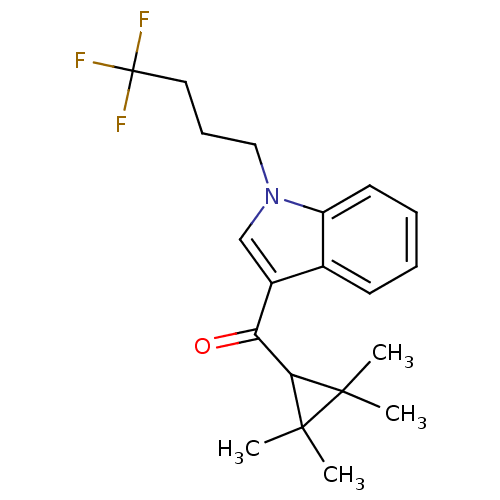
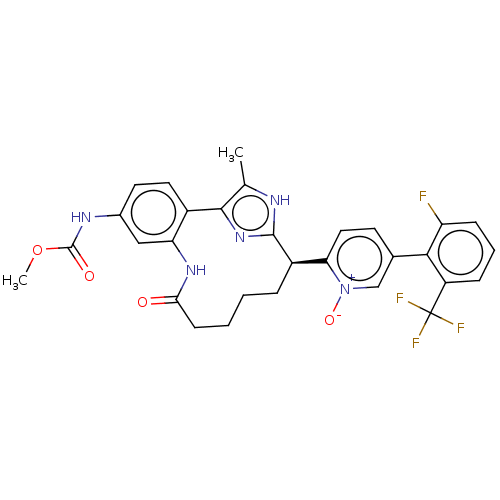
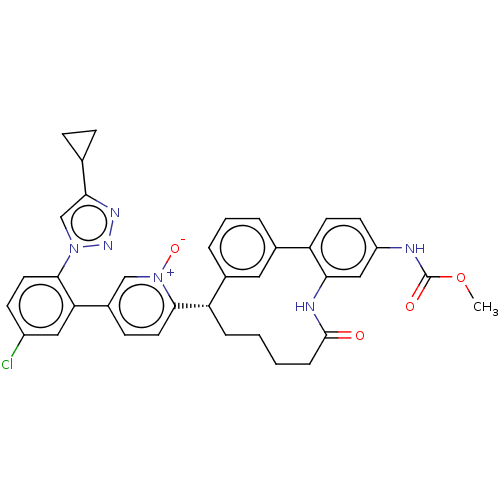
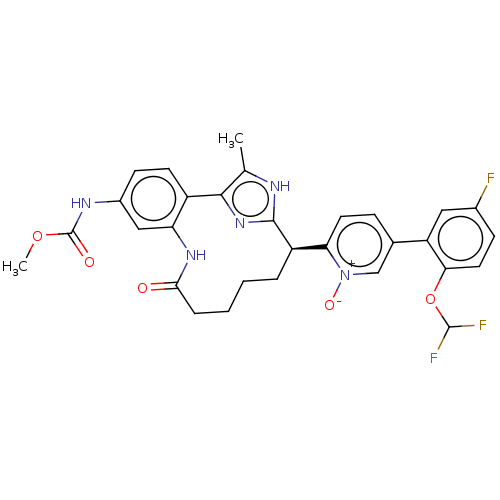
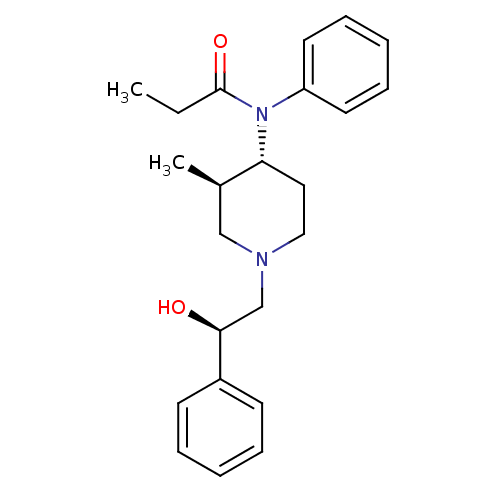
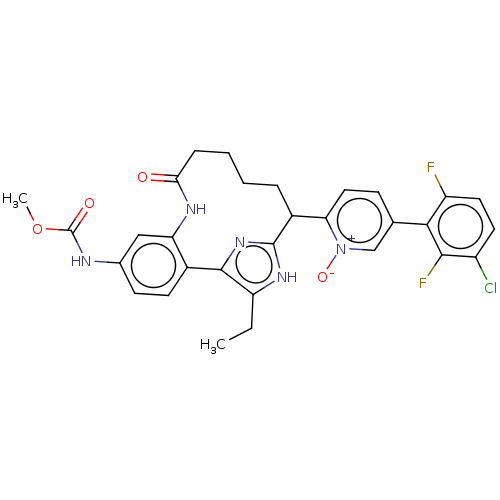
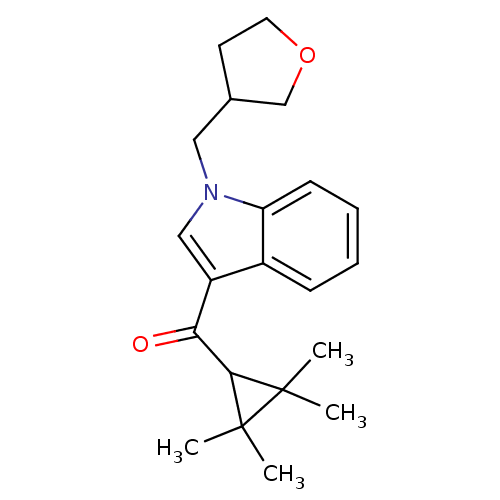
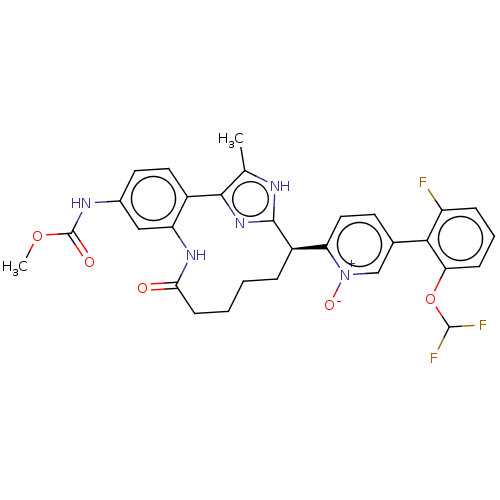
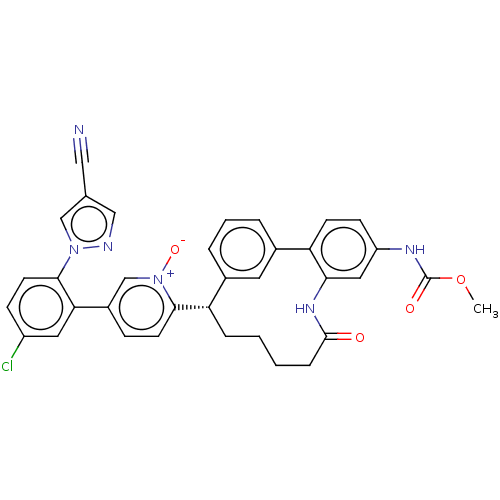
Affinity DataKi: 0.00500nMAssay Description:Inhibition against Opioid receptor mu 1 using [3H]- DAMGO radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 0.0316nMAssay Description:Displacement of [3H]N-methyl Scopolamine from human cloned muscarinic M3 receptor expressed in CHO cells by scintillation proximity assayMore data for this Ligand-Target Pair
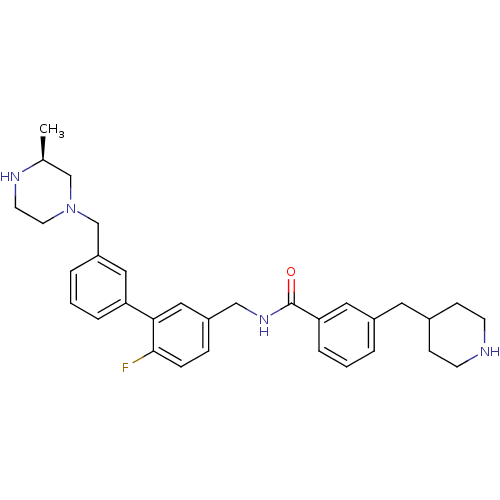
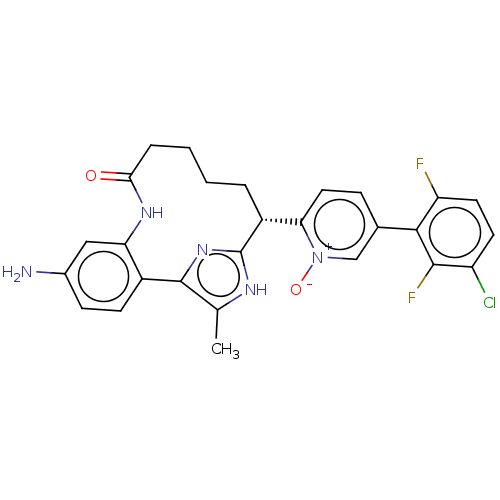
Affinity DataKi: 0.0400nMAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 0.0500nMAssay Description:Displacement of [3H]N-methyl Scopolamine from human muscarinic M1 receptor expressed in CHO cells by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.0600nMAssay Description:Inhibition against Opioid receptor mu 1 using [3H]- DAMGO radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 0.0600nMAssay Description:Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.0780nMAssay Description:Inhibition of FRET-labelled CA200645 binding to human adenosine 2A receptor expressed in CHO cells incubated for 2 hr by TR-FRET assayMore data for this Ligand-Target Pair
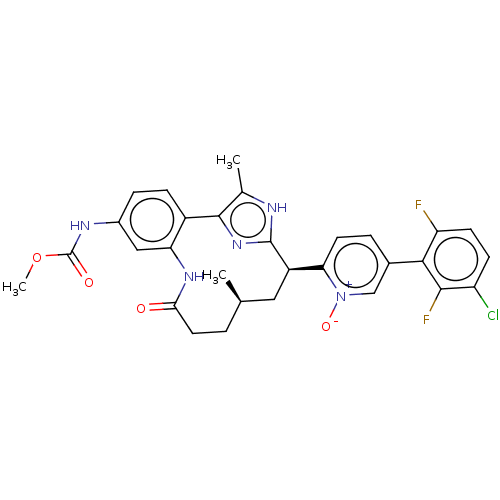
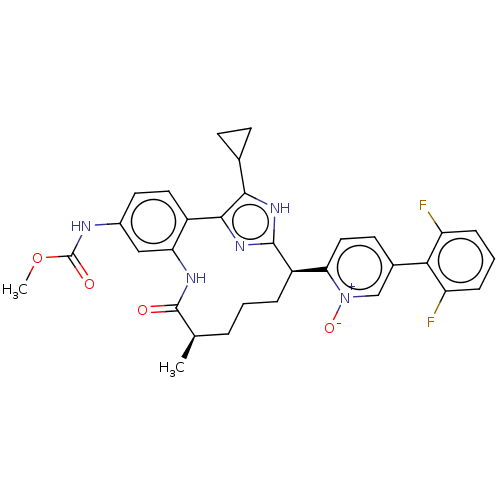
Affinity DataKi: 0.0800nMAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 0.0800nMAssay Description:Inhibition against Opioid receptor mu 1 using [3H]- DAMGO radioligand.More data for this Ligand-Target Pair
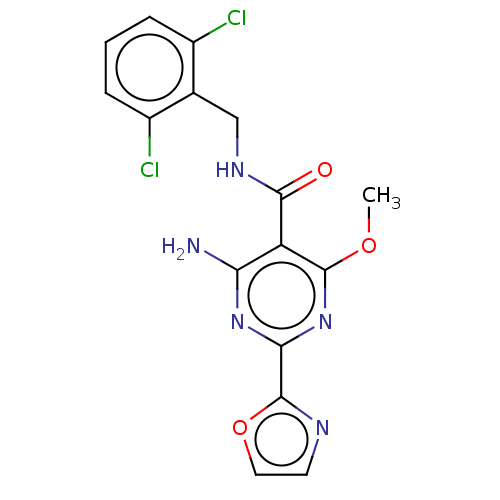
Affinity DataKi: 0.0900nMAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
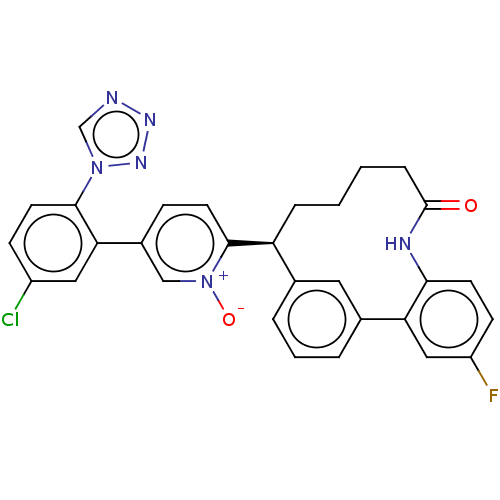
Affinity DataKi: 0.0900nMAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 0.0900nMAssay Description:Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.100nMAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 0.100nMAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 0.110nMAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 0.110nMAssay Description:Inhibition of FRET-labelled CA200645 binding to human adenosine 2A receptor expressed in CHO cells incubated for 2 hr by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.110nMAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 0.120nMAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 0.120nM EC50: >1.00E+4nMAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
Affinity DataKi: 0.130nMAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 0.130nMAssay Description:Inhibition against Opioid receptor mu 1 using [3H]- DAMGO radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 0.130nMAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 0.130nMAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 0.130nMAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 0.140nMAssay Description:Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.140nMAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 0.140nMAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 0.140nMAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 0.140nMAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 0.140nMAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 0.140nMAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 0.140nMAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 0.150nM EC50: >1.00E+4nMAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
Affinity DataKi: 0.150nMAssay Description:Inhibition against Opioid receptor mu 1 using [3H]- DAMGO radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 0.150nMAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 0.150nMAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 0.150nMAssay Description:Inhibition against Opioid receptor mu 1 using [3H]- DAMGO radioligand.More data for this Ligand-Target Pair
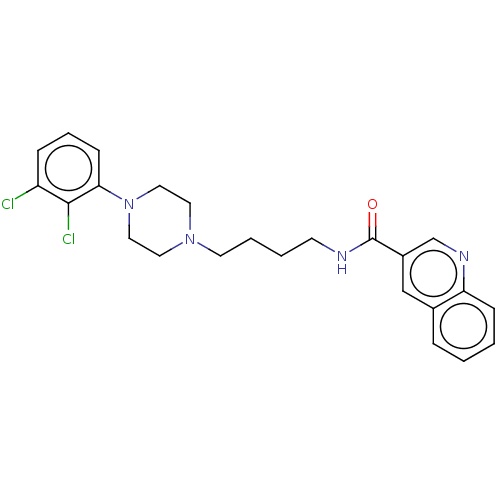
Affinity DataKi: 0.160nMAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Kallikrein can be determined using a relevant purified serine protease, a...More data for this Ligand-Target Pair
Affinity DataKi: 0.160nMAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 0.170nMAssay Description:Displacement of [3H]N-methyl Scopolamine from human muscarinic M2 receptor expressed in CHO cells by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.170nMAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 0.170nMAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 0.170nMAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 0.170nMAssay Description:Antagonist activity at 5HT2A receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 0.170nMAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
TargetD(3) dopamine receptor(Homo sapiens (Human))
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
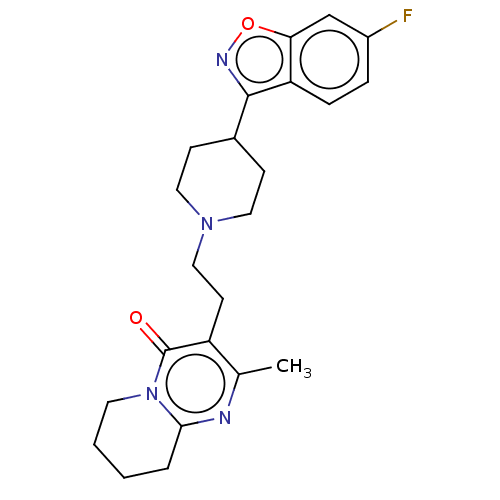
Affinity DataKi: 0.172nMAssay Description:Displacement of [3H]-N-methylspiperone from human dopamine D3 receptor expressed in HEK293 cells after 1 hr by MicroBeta microplate counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 0.180nMAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 0.180nMAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 0.180nMAssay Description:Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.190nMAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair