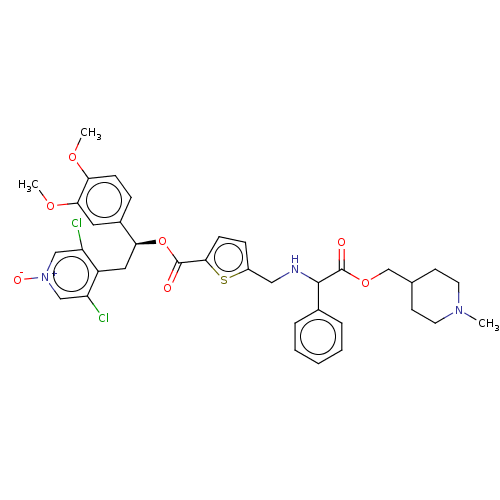
Found 9 Enz. Inhib. hit(s) with Target = 'Muscarinic acetylcholine receptor M3' and Ligand = 'BDBM325605'
Found 9 Enz. Inhib. hit(s) with Target = 'Muscarinic acetylcholine receptor M3' and Ligand = 'BDBM325605' Affinity DataKi: 0.700nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair
Affinity DataIC50: 63nMAssay Description:Antagonist activity at muscarinic M3 receptor in rat trachea assessed as inhibition of carbachol-induced contractionMore data for this Ligand-Target Pair
Affinity DataIC50: 63.1nMAssay Description:Antagonist activity at muscarinic M3 receptor in rat trachea assessed as inhibition of carbachol-induced contractionMore data for this Ligand-Target Pair