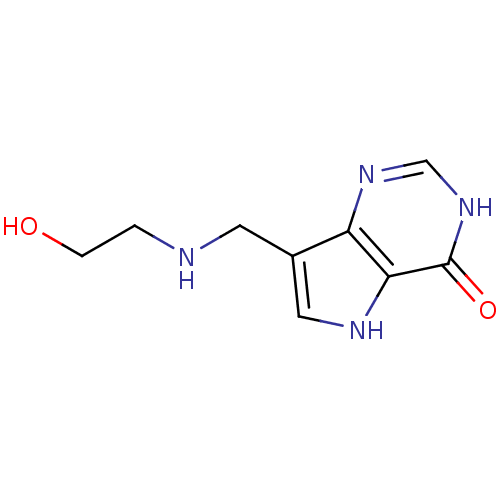
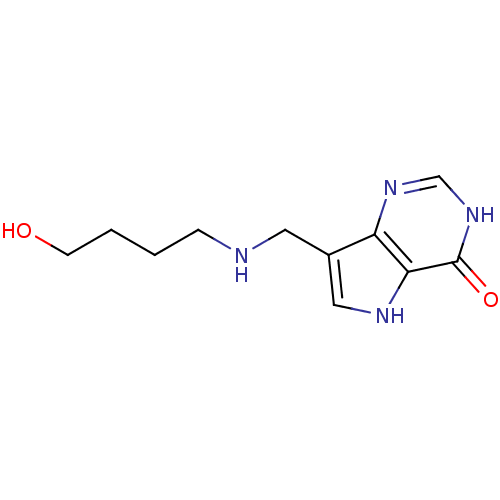
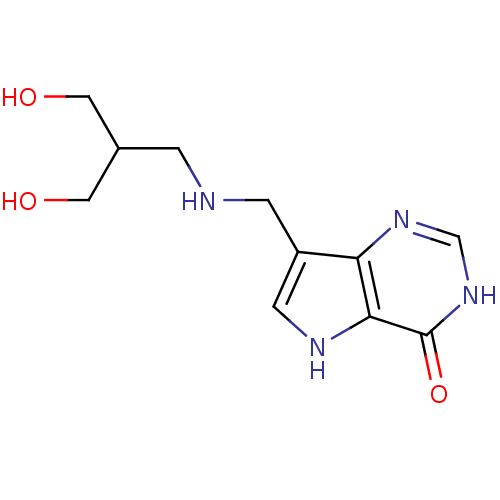
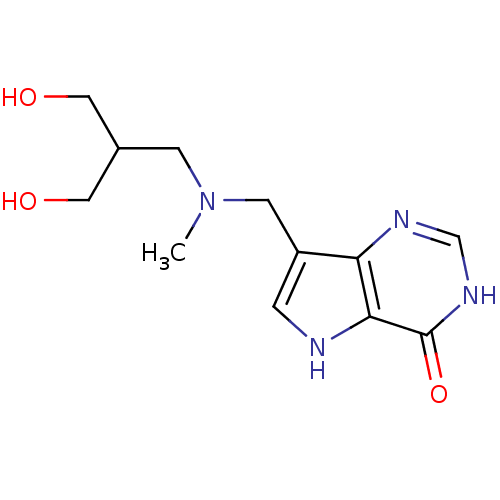
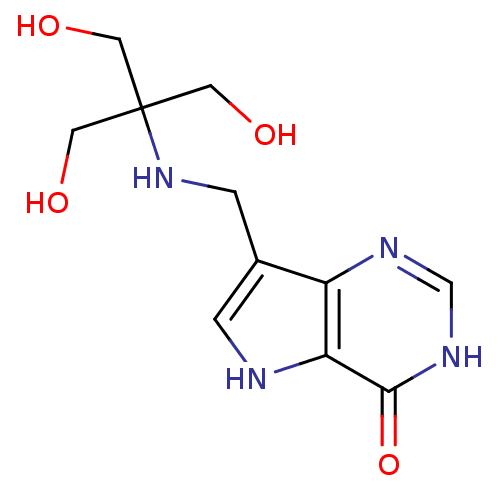
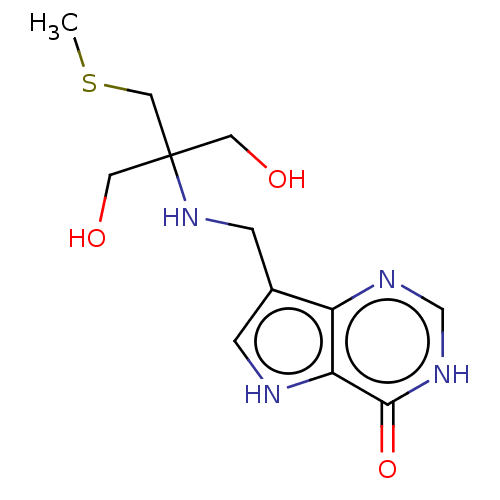
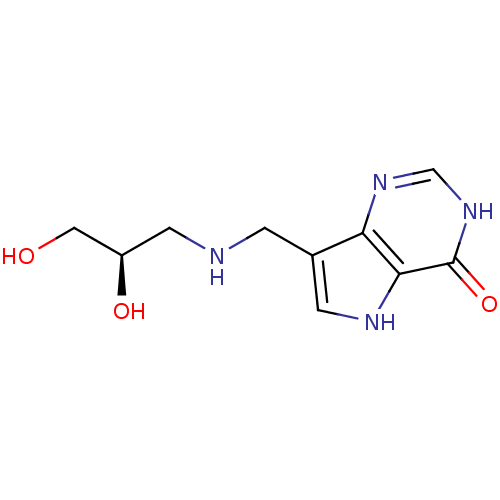
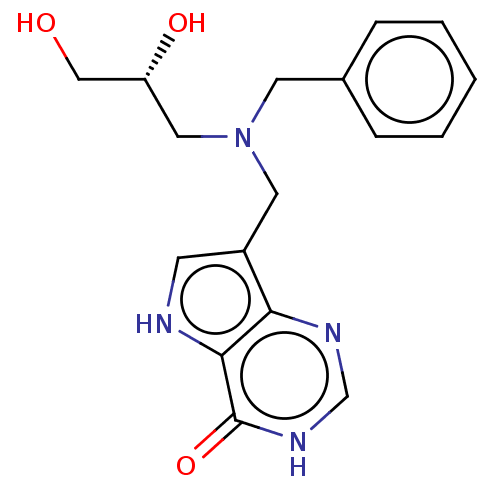
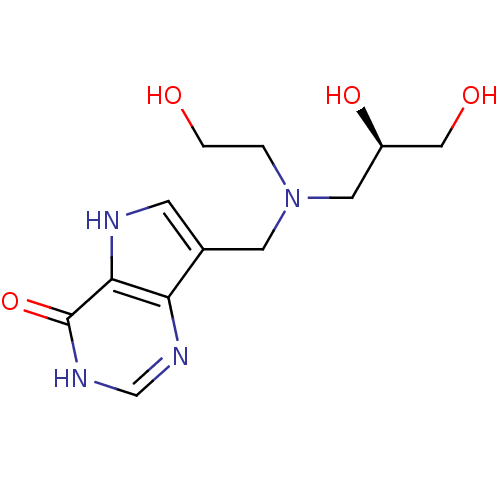
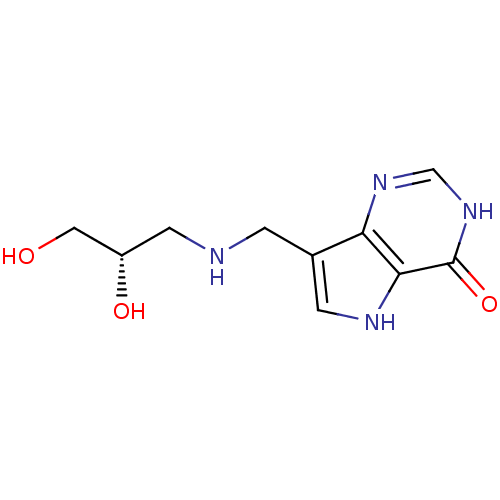
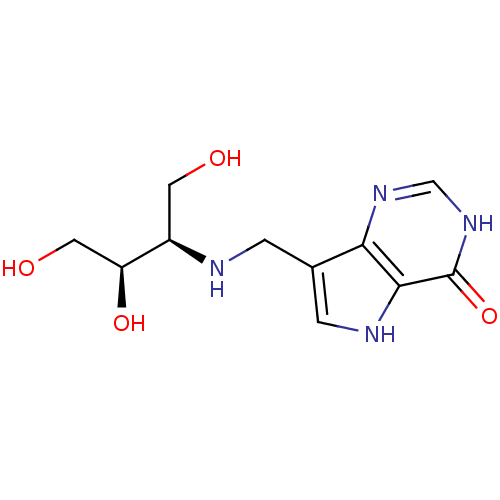
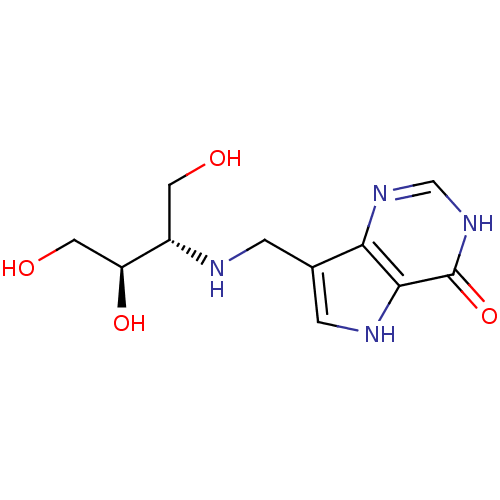
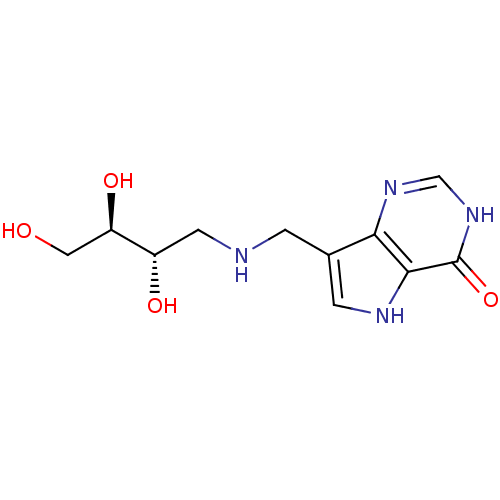
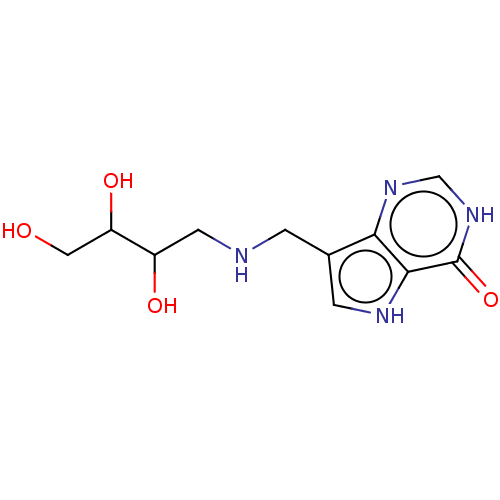
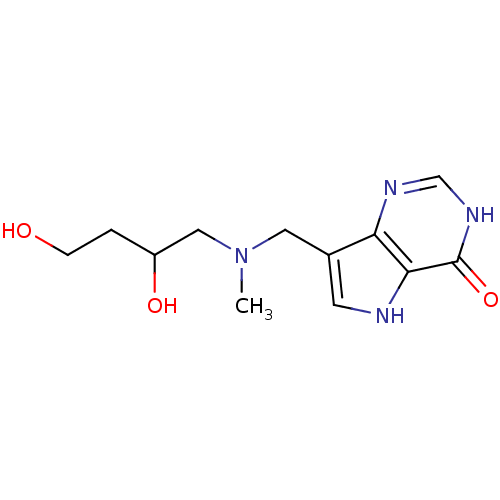
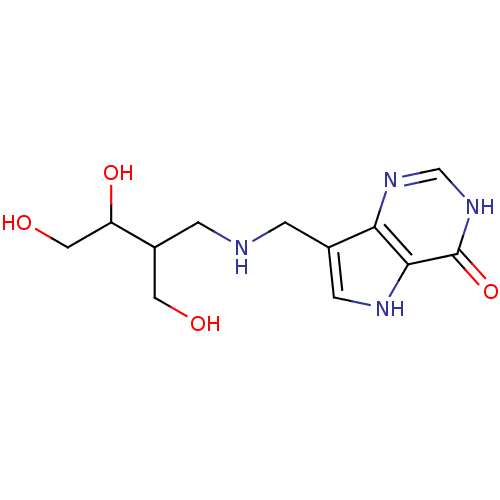
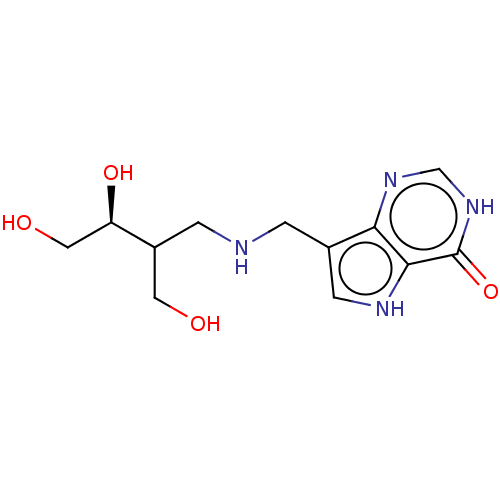
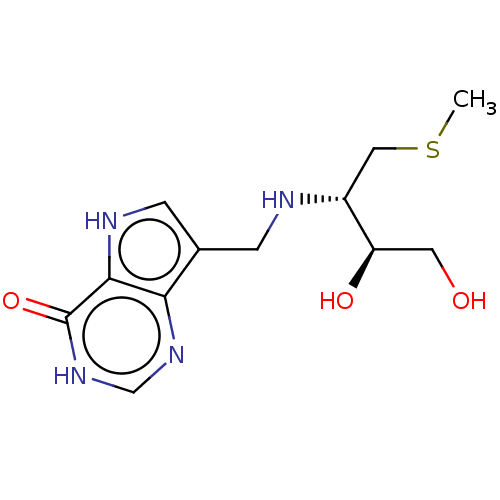
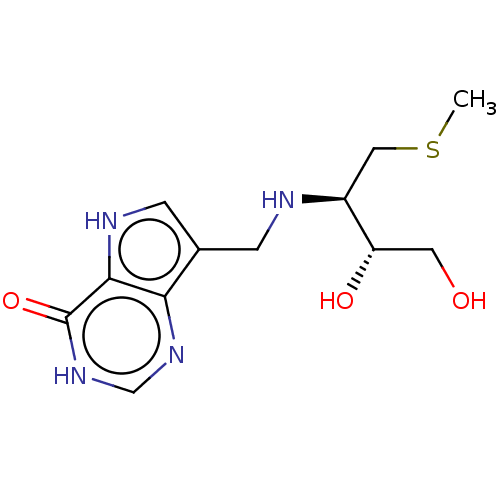
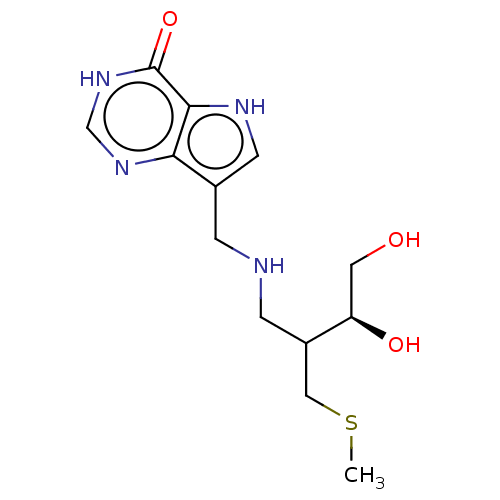
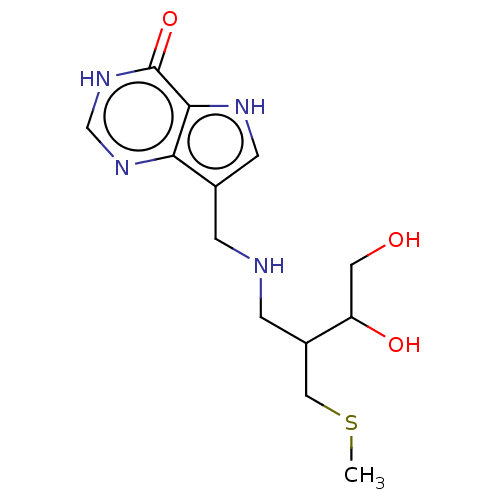
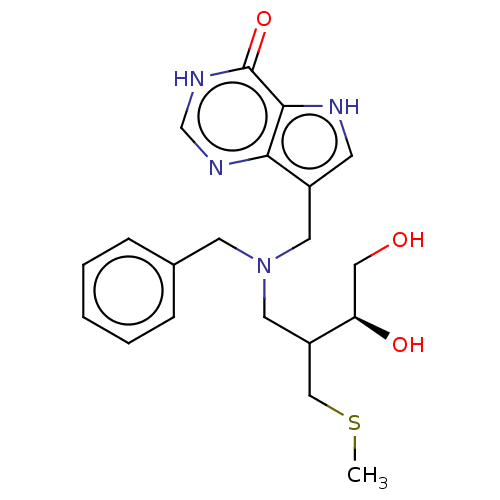
Affinity DataKd: 0.00860nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 297nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 1.10nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 25nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 14.1nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 3.70nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 0.620nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 3nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 14.9nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 300nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 165nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 4.20nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 96nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 5.20nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 4.30nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 1nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 31nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 84nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 227nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 0.780nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 900nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 15nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 74nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 71nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 142nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 5.60nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 159nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 22nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 51nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 789nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 0.210nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 430nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 770nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 210nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 2.00E+3nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 163nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 4.30E+3nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 1.00E+3nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 1.80E+3nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 2.00E+3nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 550nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 260nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 1.38E+3nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 4.80E+3nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 770nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 55nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 210nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 1.20E+4nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 3.20E+4nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 770nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair

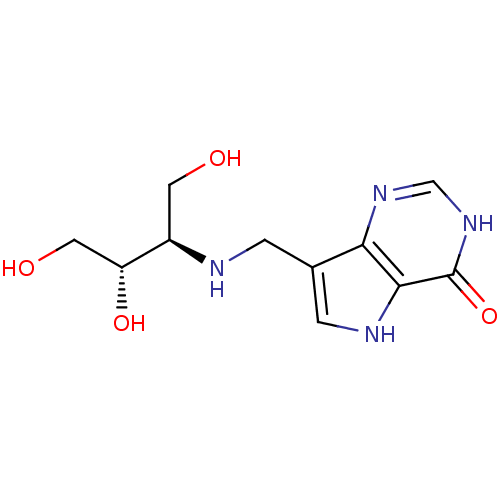
 3D Structure (crystal)
3D Structure (crystal)