Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Histone deacetylase 4
Ligand
BDBM50322421
Substrate
n/a
Meas. Tech.
ChEMBL_643211 (CHEMBL1177022)
IC50
7500±n/a nM
Citation
 Souto, JA; Vaz, E; Lepore, I; Pöppler, AC; Franci, G; Alvarez, R; Altucci, L; de Lera, AR Synthesis and biological characterization of the histone deacetylase inhibitor largazole and C7- modified analogues. J Med Chem 53:4654-67 (2010) [PubMed] Article
Souto, JA; Vaz, E; Lepore, I; Pöppler, AC; Franci, G; Alvarez, R; Altucci, L; de Lera, AR Synthesis and biological characterization of the histone deacetylase inhibitor largazole and C7- modified analogues. J Med Chem 53:4654-67 (2010) [PubMed] Article More Info.:
Target
Name:
Histone deacetylase 4
Synonyms:
Cereblon/Histone deacetylase 4 | HD4 | HDAC4 | HDAC4_HUMAN | Histone acetylase 4(HDAC4) | Human HDAC4 | KIAA0288
Type:
Enzyme
Mol. Mass.:
119049.39
Organism:
Homo sapiens (Human)
Description:
P56524
Residue:
1084
Sequence:
MSSQSHPDGLSGRDQPVELLNPARVNHMPSTVDVATALPLQVAPSAVPMDLRLDHQFSLPVAEPALREQQLQQELLALKQKQQIQRQILIAEFQRQHEQLSRQHEAQLHEHIKQQQEMLAMKHQQELLEHQRKLERHRQEQELEKQHREQKLQQLKNKEKGKESAVASTEVKMKLQEFVLNKKKALAHRNLNHCISSDPRYWYGKTQHSSLDQSSPPQSGVSTSYNHPVLGMYDAKDDFPLRKTASEPNLKLRSRLKQKVAERRSSPLLRRKDGPVVTALKKRPLDVTDSACSSAPGSGPSSPNNSSGSVSAENGIAPAVPSIPAETSLAHRLVAREGSAAPLPLYTSPSLPNITLGLPATGPSAGTAGQQDAERLTLPALQQRLSLFPGTHLTPYLSTSPLERDGGAAHSPLLQHMVLLEQPPAQAPLVTGLGALPLHAQSLVGADRVSPSIHKLRQHRPLGRTQSAPLPQNAQALQHLVIQQQHQQFLEKHKQQFQQQQLQMNKIIPKPSEPARQPESHPEETEEELREHQALLDEPYLDRLPGQKEAHAQAGVQVKQEPIESDEEEAEPPREVEPGQRQPSEQELLFRQQALLLEQQRIHQLRNYQASMEAAGIPVSFGGHRPLSRAQSSPASATFPVSVQEPPTKPRFTTGLVYDTLMLKHQCTCGSSSSHPEHAGRIQSIWSRLQETGLRGKCECIRGRKATLEELQTVHSEAHTLLYGTNPLNRQKLDSKKLLGSLASVFVRLPCGGVGVDSDTIWNEVHSAGAARLAVGCVVELVFKVATGELKNGFAVVRPPGHHAEESTPMGFCYFNSVAVAAKLLQQRLSVSKILIVDWDVHHGNGTQQAFYSDPSVLYMSLHRYDDGNFFPGSGAPDEVGTGPGVGFNVNMAFTGGLDPPMGDAEYLAAFRTVVMPIASEFAPDVVLVSSGFDAVEGHPTPLGGYNLSARCFGYLTKQLMGLAGGRIVLALEGGHDLTAICDASEACVSALLGNELDPLPEKVLQQRPNANAVRSMEKVMEIHSKYWRCLQRTTSTAGRSLIEAQTCENEEAETVTAMASLSVGVKPAEKRPDEEPMEEEPPL
Inhibitor
Name:
BDBM50322421
Synonyms:
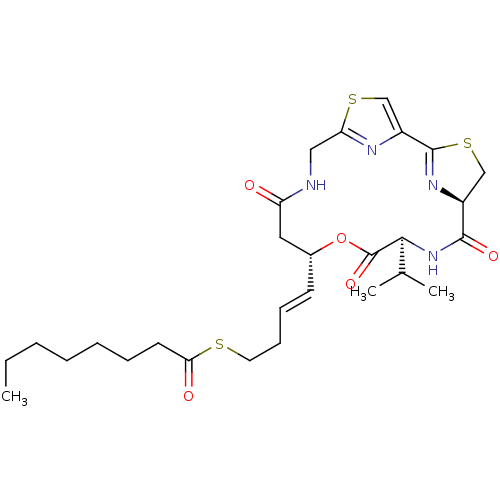
CHEMBL1173194 | Octanethioic acid S-[(E)-4-((5R,8S,11S)-8-isopropyl-6,9,13-trioxo-10-oxa-3,17-dithia-7,14,19,20-tetraaza-tricyclo[14.2.1.1*2,5*]icosa-1(18),2(20),16(19)-trien-11-yl)-but-3-enyl]ester
Type:
Small organic molecule
Emp. Form.:
C28H40N4O5S3
Mol. Mass.:
608.836
SMILES:
CCCCCCCC(=O)SCC\C=C\[C@@H]1CC(=O)NCc2nc(cs2)C2=N[C@@H](CS2)C(=O)N[C@@H](C(C)C)C(=O)O1 |r,t:26|