Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 3A4
Ligand
BDBM50190528
Substrate
n/a
Meas. Tech.
ChEMBL_821184 (CHEMBL2039726)
IC50
5000±n/a nM
Citation
 Borthwick, AD; Liddle, J; Davies, DE; Exall, AM; Hamlett, C; Hickey, DM; Mason, AM; Smith, IE; Nerozzi, F; Peace, S; Pollard, D; Sollis, SL; Allen, MJ; Woollard, PM; Pullen, MA; Westfall, TD; Stanislaus, DJ Pyridyl-2,5-diketopiperazines as potent, selective, and orally bioavailable oxytocin antagonists: synthesis, pharmacokinetics, and in vivo potency. J Med Chem 55:783-96 (2012) [PubMed] Article
Borthwick, AD; Liddle, J; Davies, DE; Exall, AM; Hamlett, C; Hickey, DM; Mason, AM; Smith, IE; Nerozzi, F; Peace, S; Pollard, D; Sollis, SL; Allen, MJ; Woollard, PM; Pullen, MA; Westfall, TD; Stanislaus, DJ Pyridyl-2,5-diketopiperazines as potent, selective, and orally bioavailable oxytocin antagonists: synthesis, pharmacokinetics, and in vivo potency. J Med Chem 55:783-96 (2012) [PubMed] Article More Info.:
Target
Name:
Cytochrome P450 3A4
Synonyms:
Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase
Type:
Enzyme
Mol. Mass.:
57349.57
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
503
Sequence:
MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMFDMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISIAEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYSMDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICVFPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSIIFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVVNETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFSKKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLGGLLQPEKPVVLKVESRDGTVSGA
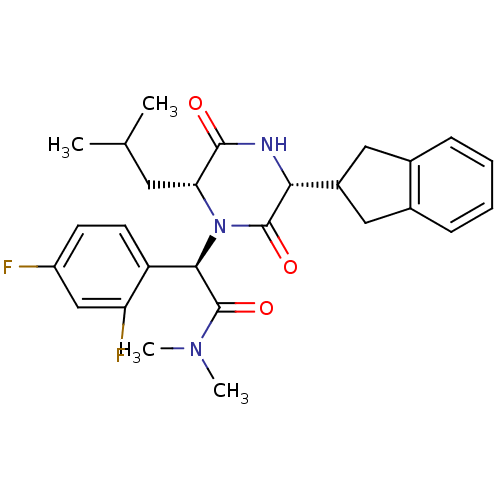
Inhibitor
Name:
BDBM50190528
Synonyms:
(2R)-2-(2,4-difluorophenyl)-2-[(3R,6R)-3-(2,3-dihydro-1H-inden-2-yl)-6-(2-methylpropyl)-2,5-dioxo-1-piperazinyl]-N,N-dimethylethanamide | CHEMBL377414
Type:
Small organic molecule
Emp. Form.:
C27H31F2N3O3
Mol. Mass.:
483.5501
SMILES:
CC(C)C[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(F)cc2F)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1