Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 3A4
Ligand
BDBM50012147
Substrate
n/a
Meas. Tech.
ChEMBL_1352055 (CHEMBL3267048)
IC50
>25000±n/a nM
Citation
 Chapman, TM; Osborne, SA; Wallace, C; Birchall, K; Bouloc, N; Jones, HM; Ansell, KH; Taylor, DL; Clough, B; Green, JL; Holder, AA Optimization of an imidazopyridazine series of inhibitors of Plasmodium falciparum calcium-dependent protein kinase 1 (PfCDPK1). J Med Chem 57:3570-87 (2014) [PubMed] Article
Chapman, TM; Osborne, SA; Wallace, C; Birchall, K; Bouloc, N; Jones, HM; Ansell, KH; Taylor, DL; Clough, B; Green, JL; Holder, AA Optimization of an imidazopyridazine series of inhibitors of Plasmodium falciparum calcium-dependent protein kinase 1 (PfCDPK1). J Med Chem 57:3570-87 (2014) [PubMed] Article More Info.:
Target
Name:
Cytochrome P450 3A4
Synonyms:
Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase
Type:
Enzyme
Mol. Mass.:
57349.57
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
503
Sequence:
MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMFDMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISIAEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYSMDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICVFPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSIIFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVVNETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFSKKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLGGLLQPEKPVVLKVESRDGTVSGA
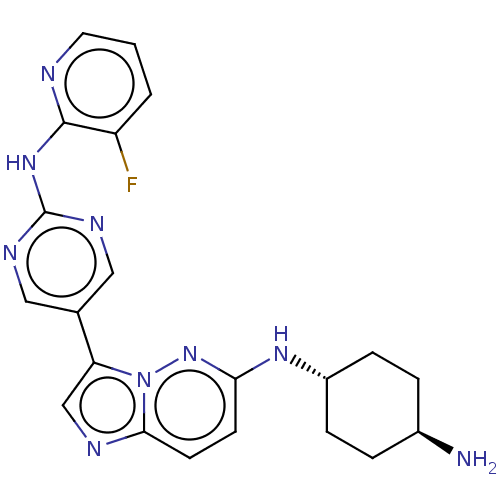
Inhibitor
Name:
BDBM50012147
Synonyms:
CHEMBL3264059
Type:
Small organic molecule
Emp. Form.:
C21H22FN9
Mol. Mass.:
419.4581
SMILES:
N[C@H]1CC[C@@H](CC1)Nc1ccc2ncc(-c3cnc(Nc4ncccc4F)nc3)n2n1 |r,wU:1.0,wD:4.7,(7.82,-13.28,;9.15,-14.06,;10.49,-13.3,;11.82,-14.07,;11.81,-15.62,;10.48,-16.38,;9.15,-15.6,;13.15,-16.39,;14.48,-15.62,;14.48,-14.07,;15.81,-13.3,;17.15,-14.07,;18.62,-13.59,;19.53,-14.84,;18.62,-16.1,;19.1,-17.56,;18.07,-18.7,;18.54,-20.16,;20.05,-20.48,;20.53,-21.95,;22.03,-22.27,;22.5,-23.73,;24.01,-24.05,;25.04,-22.9,;24.55,-21.43,;23.05,-21.12,;22.56,-19.66,;21.08,-19.33,;20.6,-17.87,;17.15,-15.62,;15.82,-16.39,)|