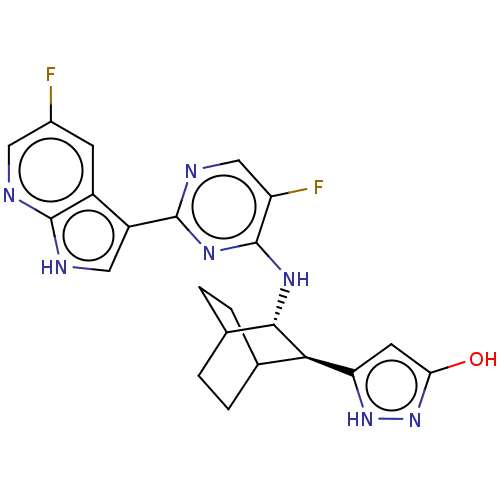
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
Ligand
BDBM50067534
Substrate
n/a
Meas. Tech.
ChEMBL_1465822 (CHEMBL3406127)
Ki
>4000±n/a nM
Citation
 Boyd, MJ; Bandarage, UK; Bennett, H; Byrn, RR; Davies, I; Gu, W; Jacobs, M; Ledeboer, MW; Ledford, B; Leeman, JR; Perola, E; Wang, T; Bennani, Y; Clark, MP; Charifson, PS Isosteric replacements of the carboxylic acid of drug candidate VX-787: Effect of charge on antiviral potency and kinase activity of azaindole-based influenza PB2 inhibitors. Bioorg Med Chem Lett 25:1990-4 (2015) [PubMed] Article
Boyd, MJ; Bandarage, UK; Bennett, H; Byrn, RR; Davies, I; Gu, W; Jacobs, M; Ledeboer, MW; Ledford, B; Leeman, JR; Perola, E; Wang, T; Bennani, Y; Clark, MP; Charifson, PS Isosteric replacements of the carboxylic acid of drug candidate VX-787: Effect of charge on antiviral potency and kinase activity of azaindole-based influenza PB2 inhibitors. Bioorg Med Chem Lett 25:1990-4 (2015) [PubMed] Article More Info.:
Target
Name:
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
Synonyms:
PI3-kinase p110 subunit beta | PI3-kinase subunit p110-beta | PI3Kbeta | PIK3C1 | PIK3CB | PK3CB_HUMAN | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta (PI3Kbeta) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (PI3K beta) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (PI3K) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (PI3K-beta) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (PI3Kbeta) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (PI3Kÿ²) | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit beta isoform | Phosphoinositide 3-Kinase (PI3K), beta | Phosphoinositide 3-Kinase (PI3K), beta Chain A | Phosphoinositide-3-kinase (PI3K beta) | PtdIns-3-kinase p110
Type:
Enzyme Subunit
Mol. Mass.:
122769.00
Organism:
Homo sapiens (Human)
Description:
P42338
Residue:
1070
Sequence:
MCFSFIMPPAMADILDIWAVDSQIASDGSIPVDFLLPTGIYIQLEVPREATISYIKQMLWKQVHNYPMFNLLMDIDSYMFACVNQTAVYEELEDETRRLCDVRPFLPVLKLVTRSCDPGEKLDSKIGVLIGKGLHEFDSLKDPEVNEFRRKMRKFSEEKILSLVGLSWMDWLKQTYPPEHEPSIPENLEDKLYGGKLIVAVHFENCQDVFSFQVSPNMNPIKVNELAIQKRLTIHGKEDEVSPYDYVLQVSGRVEYVFGDHPLIQFQYIRNCVMNRALPHFILVECCKIKKMYEQEMIAIEAAINRNSSNLPLPLPPKKTRIISHVWENNNPFQIVLVKGNKLNTEETVKVHVRAGLFHGTELLCKTIVSSEVSGKNDHIWNEPLEFDINICDLPRMARLCFAVYAVLDKVKTKKSTKTINPSKYQTIRKAGKVHYPVAWVNTMVFDFKGQLRTGDIILHSWSSFPDELEEMLNPMGTVQTNPYTENATALHVKFPENKKQPYYYPPFDKIIEKAAEIASSDSANVSSRGGKKFLPVLKEILDRDPLSQLCENEMDLIWTLRQDCREIFPQSLPKLLLSIKWNKLEDVAQLQALLQIWPKLPPREALELLDFNYPDQYVREYAVGCLRQMSDEELSQYLLQLVQVLKYEPFLDCALSRFLLERALGNRRIGQFLFWHLRSEVHIPAVSVQFGVILEAYCRGSVGHMKVLSKQVEALNKLKTLNSLIKLNAVKLNRAKGKEAMHTCLKQSAYREALSDLQSPLNPCVILSELYVEKCKYMDSKMKPLWLVYNNKVFGEDSVGVIFKNGDDLRQDMLTLQMLRLMDLLWKEAGLDLRMLPYGCLATGDRSGLIEVVSTSETIADIQLNSSNVAAAAAFNKDALLNWLKEYNSGDDLDRAIEEFTLSCAGYCVASYVLGIGDRHSDNIMVKKTGQLFHIDFGHILGNFKSKFGIKRERVPFILTYDFIHVIQQGKTGNTEKFGRFRQCCEDAYLILRRHGNLFITLFALMLTAGLPELTSVKDIQYLKDSLALGKSEEEALKQFKQKFDEALRESWTTKVNWMAHTVRKDYRS
Inhibitor
Name:
BDBM50067534
Synonyms:
CHEMBL3401985
Type:
Small organic molecule
Emp. Form.:
C22H21F2N7O
Mol. Mass.:
437.4452
SMILES:
Oc1cc([nH]n1)[C@H]1C2CCC(CC2)[C@@H]1Nc1nc(ncc1F)-c1c[nH]c2ncc(F)cc12 |r,wU:6.6,wD:13.16,(7.34,-.85,;6.12,-.67,;5.04,-1.77,;3.68,-1.07,;3.89,.44,;5.4,.7,;2.26,-1.79,;2.13,-3.4,;.73,-4.07,;-.57,-3.2,;-.48,-1.66,;.83,-1.64,;.86,-3.36,;.93,-.96,;.98,.59,;-.32,1.41,;-.27,2.95,;-1.58,3.76,;-2.94,3.04,;-2.99,1.5,;-1.68,.69,;-1.73,-.55,;-1.52,5.3,;-2.72,6.2,;-2.21,7.66,;-.67,7.61,;.39,8.73,;1.9,8.35,;2.32,6.88,;3.52,6.58,;1.25,5.75,;-.25,6.14,)|