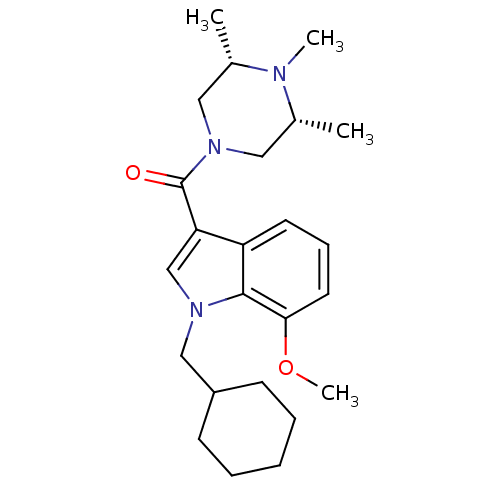
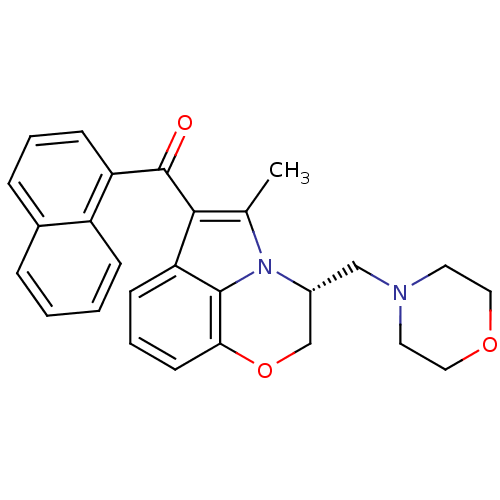
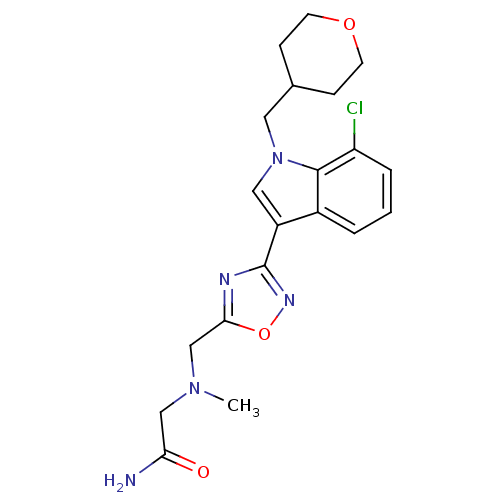
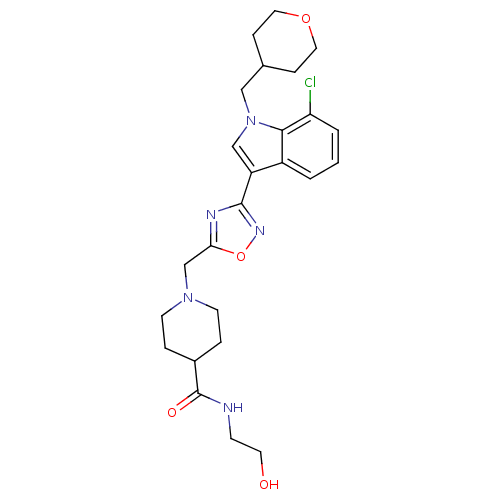
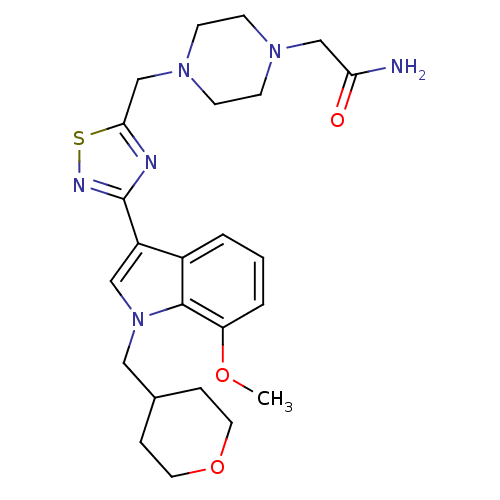
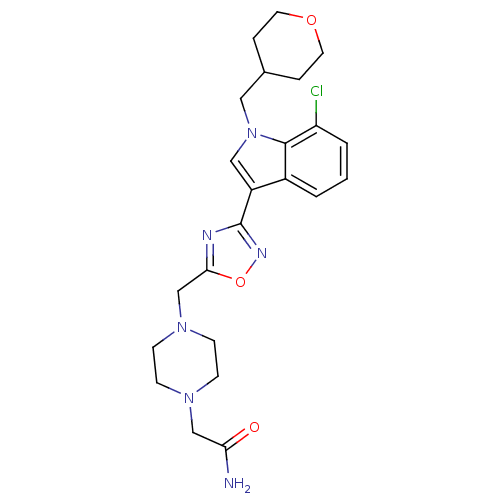
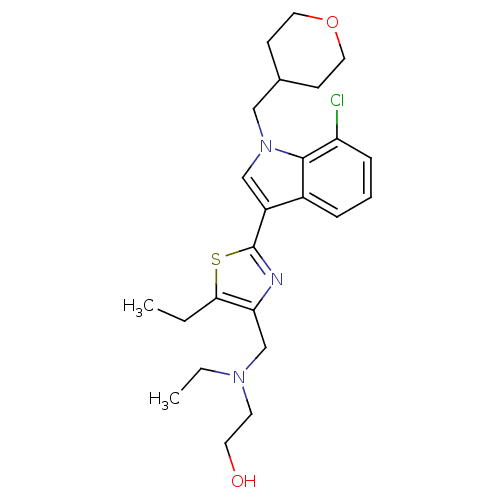
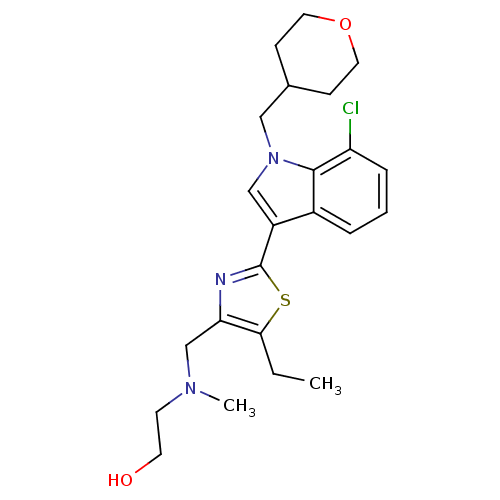
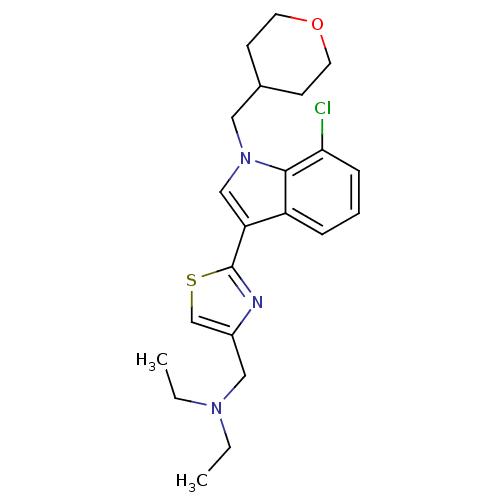
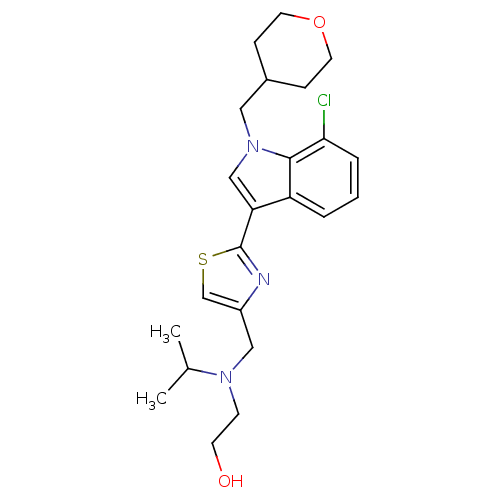
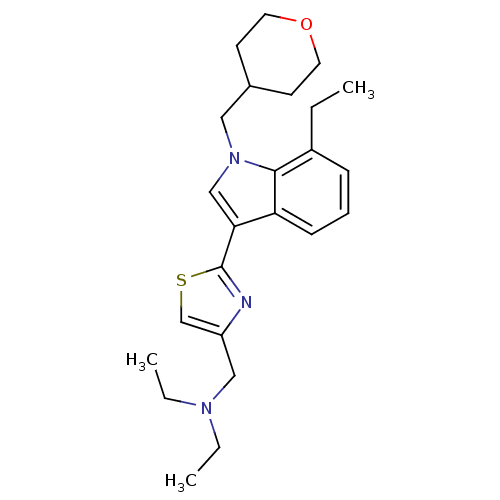
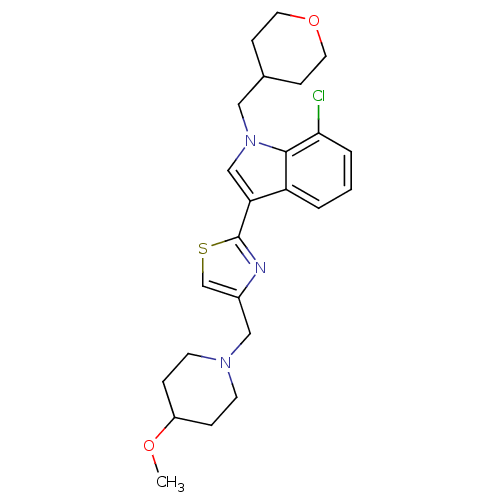
Affinity DataKi: 0.316nMAssay Description:Displacement of [3H]CP55940 from human cannabinoid CB2 receptor expressed in insect Sf9 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.316nMAssay Description:Displacement of [3H]CP55940 from human cannabinoid CB1 receptor expressed in insect Sf9 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.794nMAssay Description:Displacement of [3H]CP55940 from human cannabinoid CB2 receptor expressed in insect Sf9 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.26nMAssay Description:Displacement of [3H]CP55940 from human cannabinoid CB1 receptor expressed in insect Sf9 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2.51nMAssay Description:Displacement of [3H]CP55940 from human cannabinoid CB2 receptor expressed in insect Sf9 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 3.98nMAssay Description:Displacement of [3H]CP55940 from human cannabinoid CB1 receptor expressed in insect Sf9 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 3.98nMAssay Description:Displacement of [3H]CP 55,940 from human CB1 receptor expressed in insect sf9 membranesMore data for this Ligand-Target Pair
Affinity DataKi: 6.31nMAssay Description:Displacement of [3H]CP 55,940 from human CB1 receptor expressed in insect sf9 membranesMore data for this Ligand-Target Pair
Affinity DataKi: 6.31nMAssay Description:Displacement of [3H]CP55940 from human cannabinoid CB2 receptor expressed in insect Sf9 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 12.6nMAssay Description:Displacement of [3H]CP55940 from human cannabinoid CB1 receptor expressed in insect Sf9 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 15.8nMAssay Description:Displacement of [3H]CP 55,940 from human CB2 receptor expressed in insect sf9 membranesMore data for this Ligand-Target Pair
Affinity DataKi: 19.9nMAssay Description:Displacement of [3H]CP 55,940 from human CB2 receptor expressed in insect sf9 membranesMore data for this Ligand-Target Pair
Affinity DataKi: 19.9nMAssay Description:Displacement of [3H]CP 55,940 from human CB1 receptor expressed in insect sf9 membranesMore data for this Ligand-Target Pair
Affinity DataKi: 19.9nMAssay Description:Displacement of [3H]CP 55,940 from human CB2 receptor expressed in insect sf9 membranesMore data for this Ligand-Target Pair
Affinity DataKi: 31.6nMAssay Description:Displacement of [3H]CP 55,940 from human CB1 receptor expressed in insect sf9 membranesMore data for this Ligand-Target Pair
Affinity DataKi: 50.1nMAssay Description:Displacement of [3H]CP 55,940 from human CB2 receptor expressed in insect sf9 membranesMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 251nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 251nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 316nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 631nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 631nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 631nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 794nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 794nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 794nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 1.00E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 1.00E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 1.00E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 1.26E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 1.58E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 1.58E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 2.00E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 2.51E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 2.51E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 3.98E+3nMAssay Description:Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 2.00E+4nMAssay Description:Displacement of [3H]dofetilide from human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 3.16E+4nMAssay Description:Displacement of [35S]MK499 from human ERG channel in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of CYP1A1More data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: 3.30E+3nMAssay Description:Inhibition of CYP2C8More data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibition of human CYP3A4More data for this Ligand-Target Pair
Affinity DataIC50: 7.20E+3nMAssay Description:Inhibition of CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of human CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+4nMAssay Description:Inhibition of human CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 9.78E+4nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of CYP2E1More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of CYP3A4 using dibenzylfluorescein as a substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of CYP3A4 using 7-benzyloxyquinoline as a substrateMore data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)