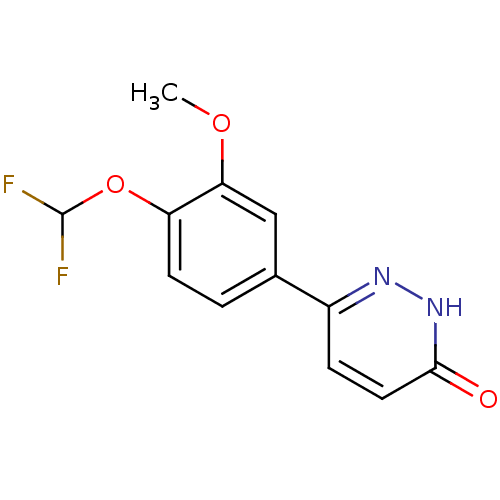
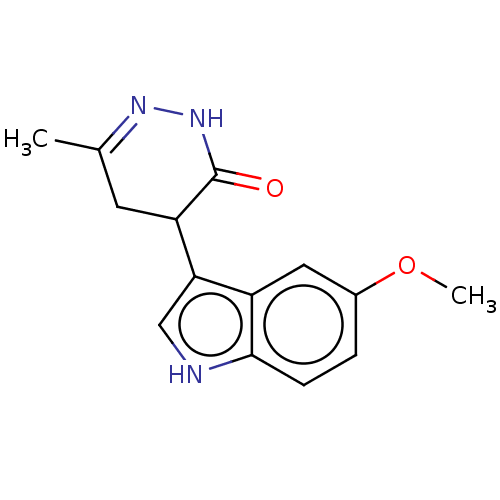
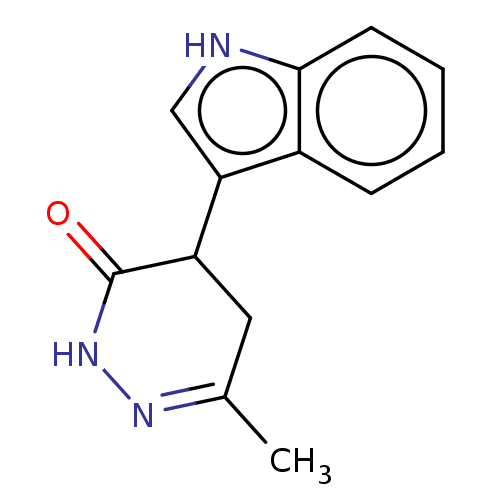
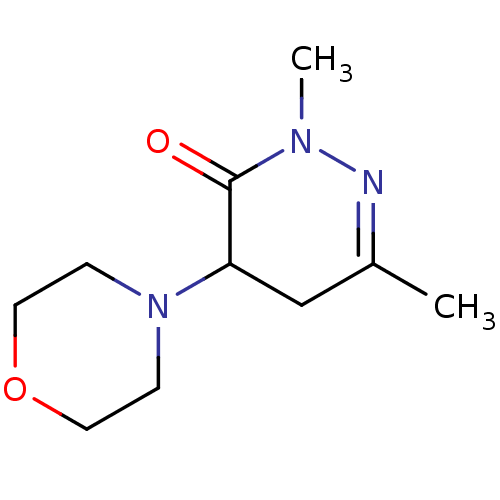
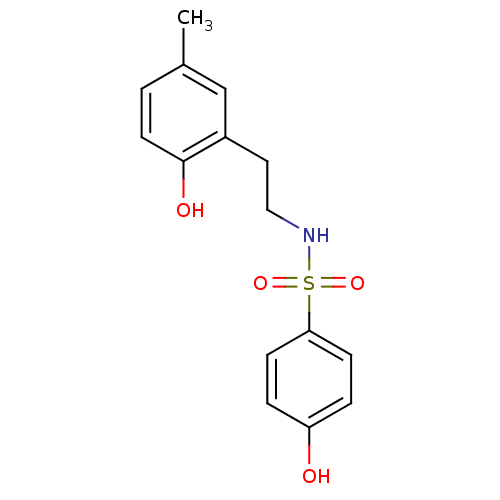
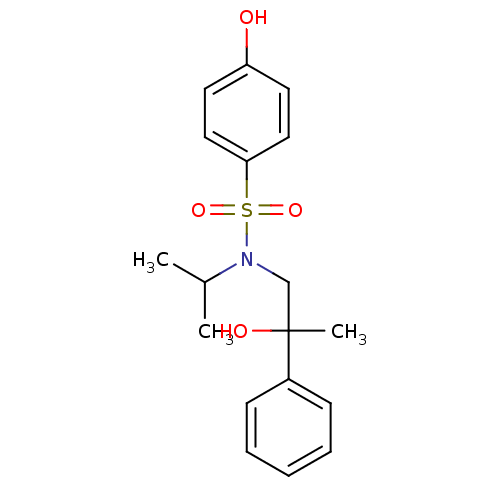
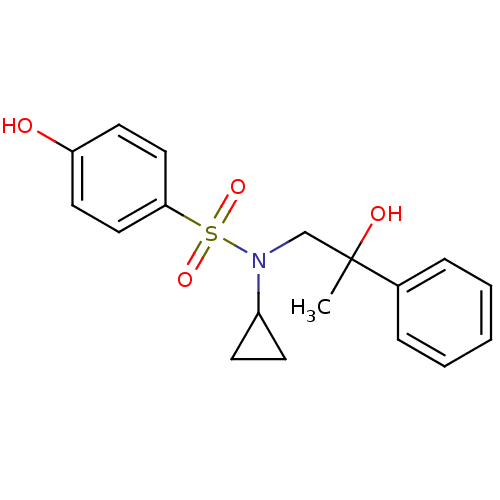
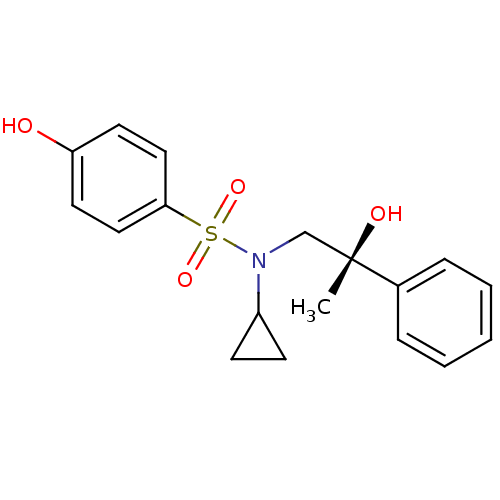
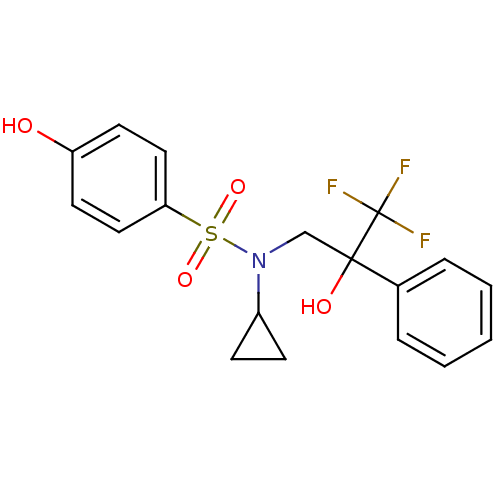
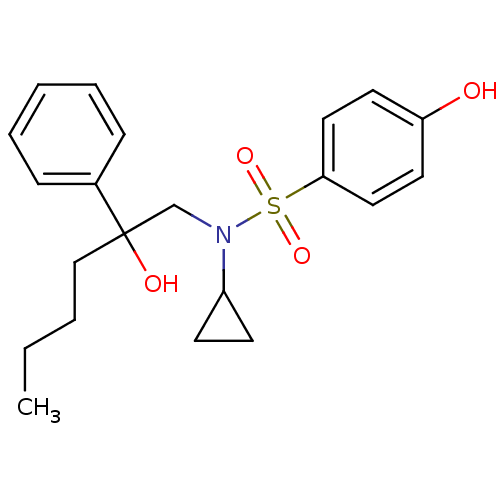
Affinity DataKi: 0.700nMAssay Description:Inhibition of [3H]-L-364,718 binding to cholecystokinin type A receptor in rat pancreas membranesMore data for this Ligand-Target Pair
Affinity DataKi: 49nMAssay Description:Inhibition of [3H]-L-364,718 binding to cholecystokinin type A receptor in rat pancreas membranesMore data for this Ligand-Target Pair
Affinity DataKi: 90nMAssay Description:Inhibition of [3H]-L-364,718 binding to cholecystokinin type A receptor in rat pancreas membranesMore data for this Ligand-Target Pair
Affinity DataKi: 180nMAssay Description:Displacement of [3H]-L-364,718 from Cholecystokinin type A receptor of rat pancreatic membranesMore data for this Ligand-Target Pair
Affinity DataKi: 200nMAssay Description:Displacement of [3H]-L-364,718 from Cholecystokinin type A receptor of rat pancreatic membranesMore data for this Ligand-Target Pair
Affinity DataKi: 200nMAssay Description:Displacement of [3H]-L-364,718 from Cholecystokinin type A receptor of rat pancreatic membranesMore data for this Ligand-Target Pair
Affinity DataKi: 290nMAssay Description:Displacement of [3H]-L-364,718 from Cholecystokinin type A receptor of rat pancreatic membranesMore data for this Ligand-Target Pair
Affinity DataKi: 340nMAssay Description:Displacement of [3H]-L-364,718 from Cholecystokinin type A receptor of rat pancreatic membranesMore data for this Ligand-Target Pair
Affinity DataKi: 400nMAssay Description:Displacement of [3H]-L-364,718 from Cholecystokinin type A receptor of rat pancreatic membranesMore data for this Ligand-Target Pair
Affinity DataKi: 400nMAssay Description:Inhibition of [3H]-L-364,718 binding to cholecystokinin type A receptor in rat pancreas membranesMore data for this Ligand-Target Pair
Affinity DataKi: 450nMAssay Description:Inhibition of [3H]-L-364,718 binding to cholecystokinin type A receptor in rat pancreas membranesMore data for this Ligand-Target Pair
Affinity DataKi: 470nMAssay Description:Displacement of [3H]-L-364,718 from Cholecystokinin type A receptor of rat pancreatic membranesMore data for this Ligand-Target Pair
Affinity DataKi: 800nMAssay Description:Inhibition of [3H]-L-364,718 binding to cholecystokinin type A receptor in rat pancreas membranesMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nMAssay Description:Inhibition of [3H]-L-364,718 binding to cholecystokinin type A receptor in rat pancreas membranesMore data for this Ligand-Target Pair
Affinity DataKi: 1.40E+3nMAssay Description:Displacement of [3H]-L-364,718 from Cholecystokinin type A receptor of rat pancreatic membranesMore data for this Ligand-Target Pair
Affinity DataKi: >2.00E+3nMAssay Description:Displacement of [3H]-L-364,718 from Cholecystokinin type A receptor of rat pancreatic membranesMore data for this Ligand-Target Pair
Affinity DataKi: >2.00E+3nMAssay Description:Displacement of [3H]-L-364,718 from Cholecystokinin type A receptor of rat pancreatic membranesMore data for this Ligand-Target Pair
Affinity DataKi: >2.00E+3nMAssay Description:Compound was evaluated for its ability to inhibit [3H]-L-364,718 binding Cholecystokinin type A receptor in rat pancreas membranesMore data for this Ligand-Target Pair
Affinity DataKi: >2.00E+3nMAssay Description:Inhibition of [3H]-L-364,718 binding to cholecystokinin type A receptor in rat pancreas membranesMore data for this Ligand-Target Pair
Affinity DataKi: >2.00E+3nMAssay Description:Displacement of [3H]-L-364,718 from Cholecystokinin type A receptor of rat pancreatic membranesMore data for this Ligand-Target Pair
Affinity DataKi: >4.00E+3nMAssay Description:Inhibition of [3H]-L-364,718 binding to cholecystokinin type A receptor in rat pancreas membranesMore data for this Ligand-Target Pair
Affinity DataKi: >4.00E+3nMAssay Description:Displacement of [3H]-L-364,718 from Cholecystokinin type A receptor of rat pancreatic membranesMore data for this Ligand-Target Pair
Affinity DataKi: >4.00E+3nMAssay Description:Displacement of [3H]-L-364,718 from Cholecystokinin type A receptor of rat pancreatic membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMpH: 7.4 T: 2°CAssay Description:A Lance TR-FRET assay (Perkin Elmer) was used to measure and compare the potency of compounds against Syk kinase domain.More data for this Ligand-Target Pair
Affinity DataIC50: 100nMpH: 7.4 T: 2°CAssay Description:A Lance TR-FRET assay (Perkin Elmer) was used to measure and compare the potency of compounds against Syk kinase domain.More data for this Ligand-Target Pair
Affinity DataIC50: 320nMpH: 7.4 T: 2°CAssay Description:A Lance TR-FRET assay (Perkin Elmer) was used to measure and compare the potency of compounds against Syk kinase domain.More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4D(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of recombinant human PDE4D expressed in Escherichia coli assessed as increase in cAMP levels after 60 mins by BIOMOLGREEN dye-based assayMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4D(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataIC50: 2.30E+4nMAssay Description:Inhibition of recombinant human PDE4D expressed in Escherichia coli assessed as increase in cAMP levels after 60 mins by BIOMOLGREEN dye-based assayMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4D(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataIC50: 3.20E+4nMAssay Description:Inhibition of recombinant human PDE4D expressed in Escherichia coli assessed as increase in cAMP levels after 60 mins by BIOMOLGREEN dye-based assayMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4D(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataIC50: 3.50E+4nMAssay Description:Inhibition of recombinant human PDE4D expressed in Escherichia coli assessed as increase in cAMP levels after 60 mins by BIOMOLGREEN dye-based assayMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4D(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataIC50: 3.80E+4nMAssay Description:Inhibition of recombinant human PDE4D expressed in Escherichia coli assessed as increase in cAMP levels after 60 mins by BIOMOLGREEN dye-based assayMore data for this Ligand-Target Pair
Affinity DataEC50: 3.50E+3nMAssay Description:Agonist activity at ERalpha expressed in HEK cells co-expressing beta-lactamase after 18 hrs by beta-lactamase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: >2.40E+3nMAssay Description:Agonist activity at ERbeta expressed in UAS cells co-expressing beta-lactamase after 18 hrs by beta-lactamase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 1.10E+3nMAssay Description:Agonist activity at ERbeta expressed in UAS cells co-expressing beta-lactamase after 18 hrs by beta-lactamase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: >3.20E+3nMAssay Description:Agonist activity at ERalpha expressed in HEK cells co-expressing beta-lactamase after 18 hrs by beta-lactamase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 418nMAssay Description:Agonist activity at ERbeta expressed in UAS cells co-expressing beta-lactamase after 18 hrs by beta-lactamase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: >2.61E+3nMAssay Description:Agonist activity at ERalpha expressed in HEK cells co-expressing beta-lactamase after 18 hrs by beta-lactamase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 84nMAssay Description:Agonist activity at ERbeta expressed in UAS cells co-expressing beta-lactamase after 18 hrs by beta-lactamase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: >1.10E+3nMAssay Description:Agonist activity at ERalpha expressed in HEK cells co-expressing beta-lactamase after 18 hrs by beta-lactamase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 101nMAssay Description:Agonist activity at ERbeta expressed in UAS cells co-expressing beta-lactamase after 18 hrs by beta-lactamase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 2.31E+3nMAssay Description:Agonist activity at ERalpha expressed in HEK cells co-expressing beta-lactamase after 18 hrs by beta-lactamase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 79nMAssay Description:Agonist activity at ERbeta expressed in UAS cells co-expressing beta-lactamase after 18 hrs by beta-lactamase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: >5.40E+3nMAssay Description:Agonist activity at ERalpha expressed in HEK cells co-expressing beta-lactamase after 18 hrs by beta-lactamase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 110nMAssay Description:Agonist activity at ERbeta expressed in UAS cells co-expressing beta-lactamase after 18 hrs by beta-lactamase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: >4.56E+3nMAssay Description:Agonist activity at ERalpha expressed in HEK cells co-expressing beta-lactamase after 18 hrs by beta-lactamase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 14nMAssay Description:Agonist activity at ERbeta expressed in UAS cells co-expressing beta-lactamase after 18 hrs by beta-lactamase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: >2.68E+3nMAssay Description:Agonist activity at ERalpha expressed in HEK cells co-expressing beta-lactamase after 18 hrs by beta-lactamase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 65nMAssay Description:Agonist activity at ERbeta expressed in UAS cells co-expressing beta-lactamase after 18 hrs by beta-lactamase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: >6.51E+3nMAssay Description:Agonist activity at ERalpha expressed in HEK cells co-expressing beta-lactamase after 18 hrs by beta-lactamase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 48nMAssay Description:Agonist activity at ERbeta expressed in UAS cells co-expressing beta-lactamase after 18 hrs by beta-lactamase reporter gene assayMore data for this Ligand-Target Pair

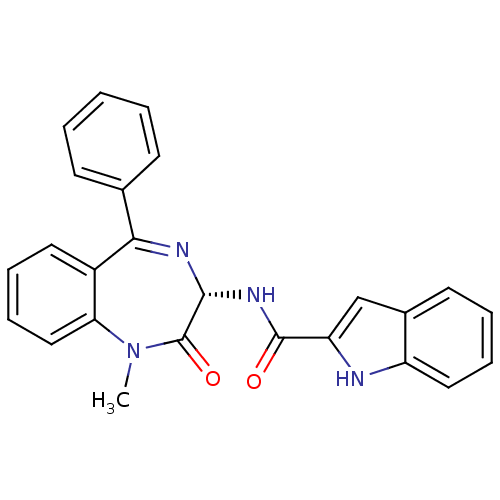

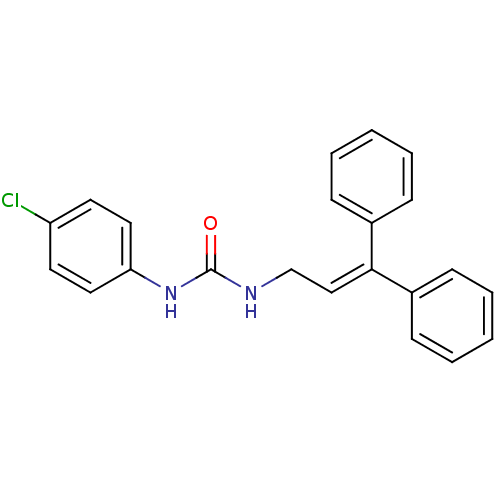
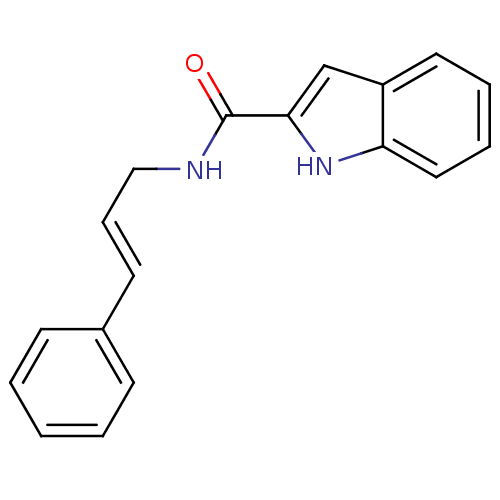



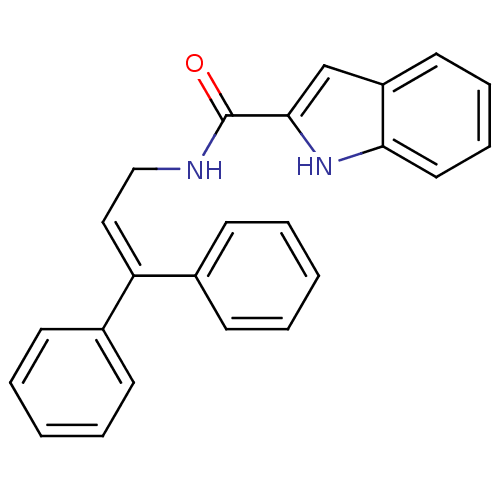
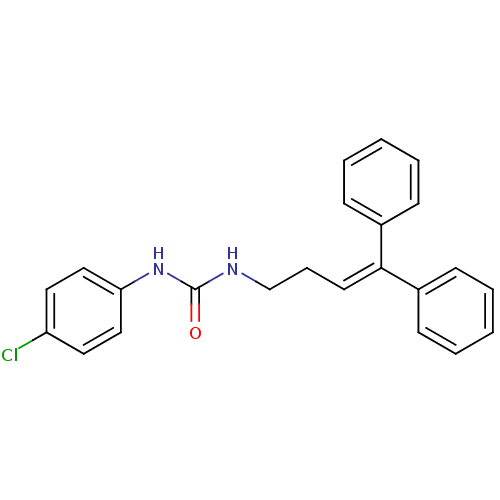


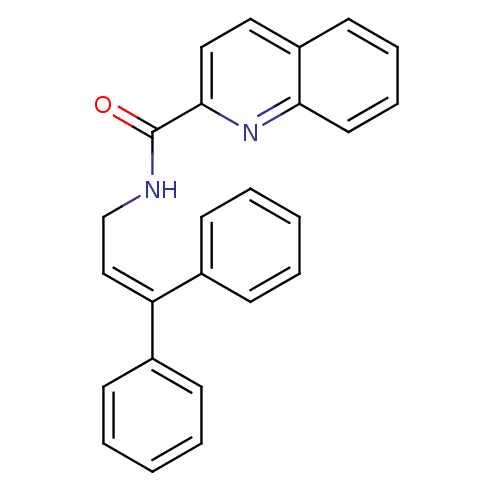
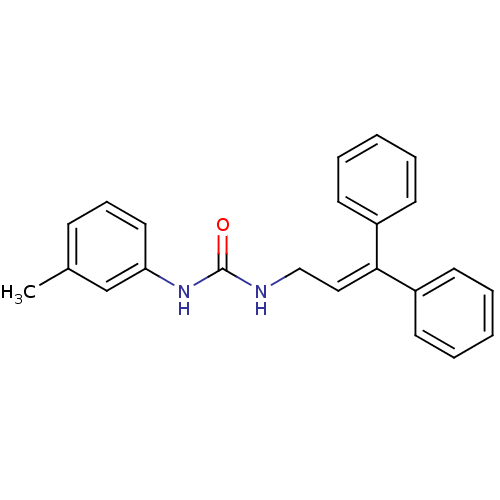
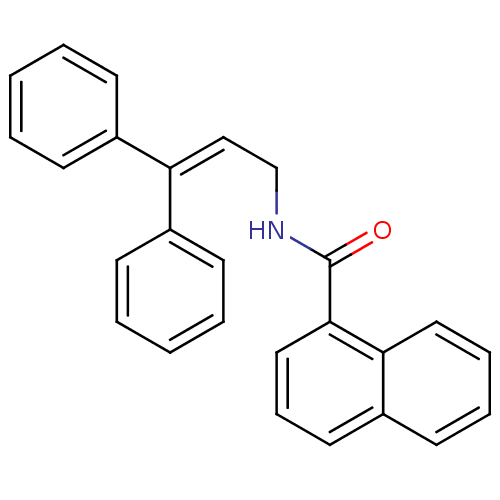

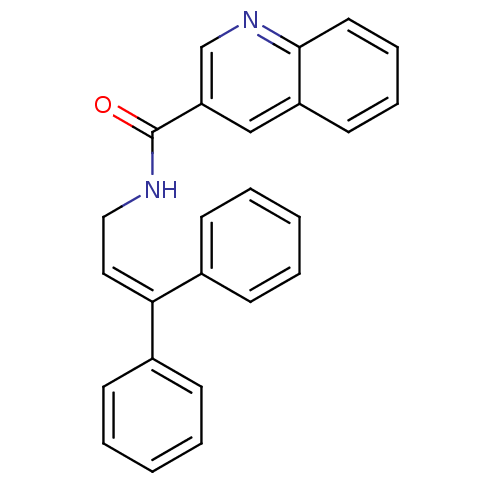
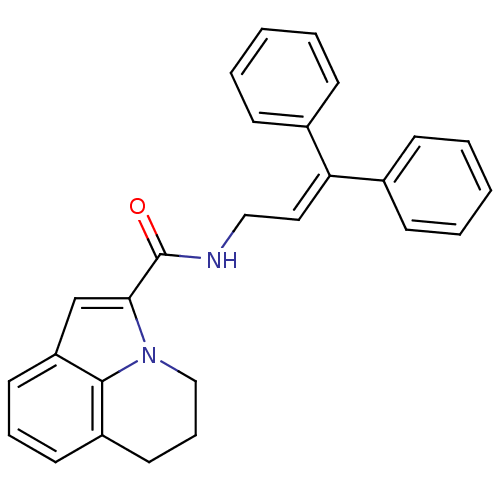




 3D Structure (crystal)
3D Structure (crystal)