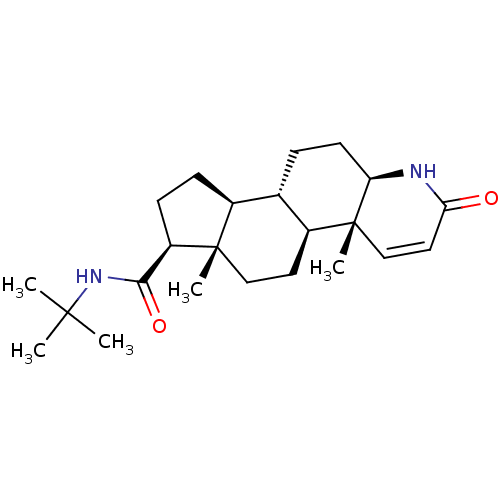
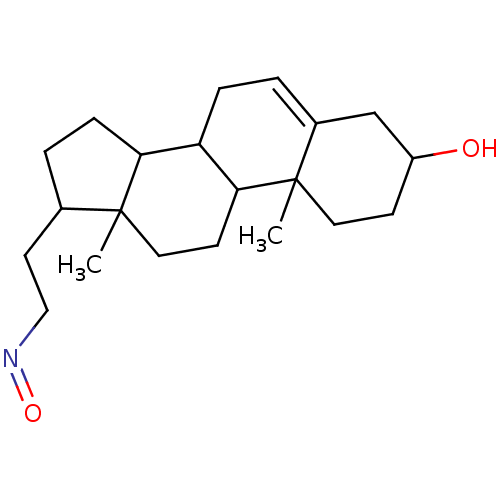
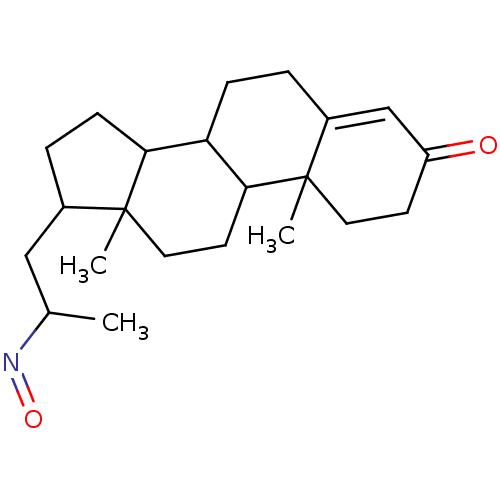
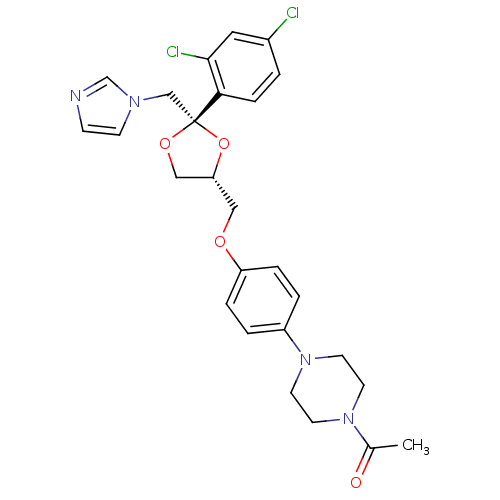
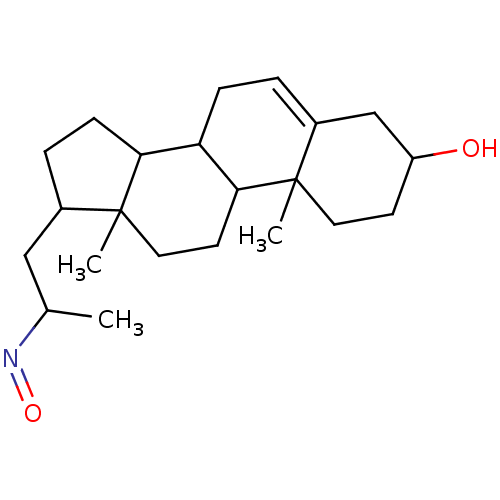
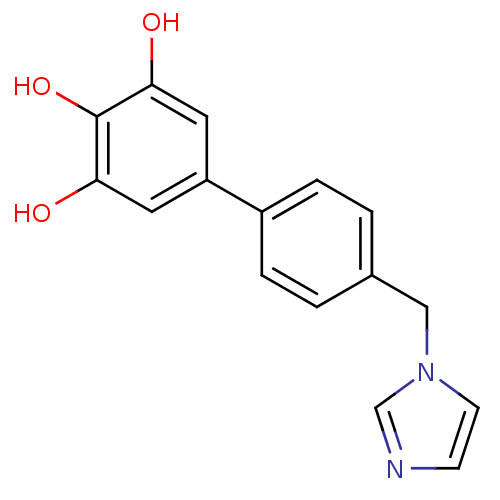
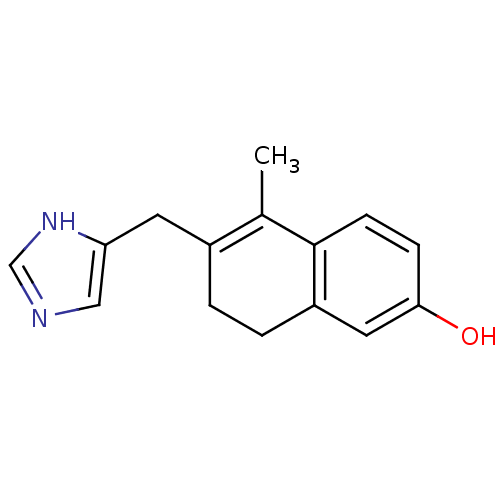
Target3-oxo-5-alpha-steroid 4-dehydrogenase 2(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type 2 from human BPH tissue at 210 nM of testosteroneMore data for this Ligand-Target Pair
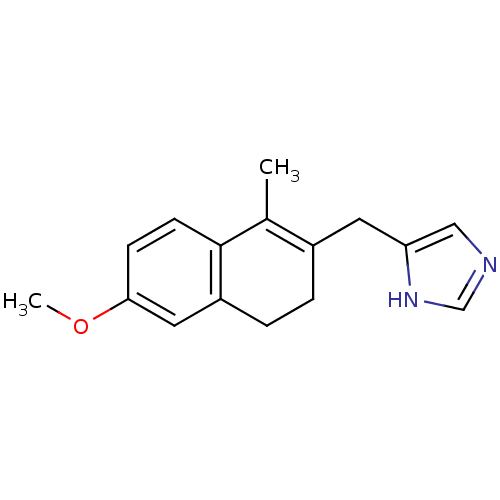
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Inhibition of human Cytochrome P450 17More data for this Ligand-Target Pair
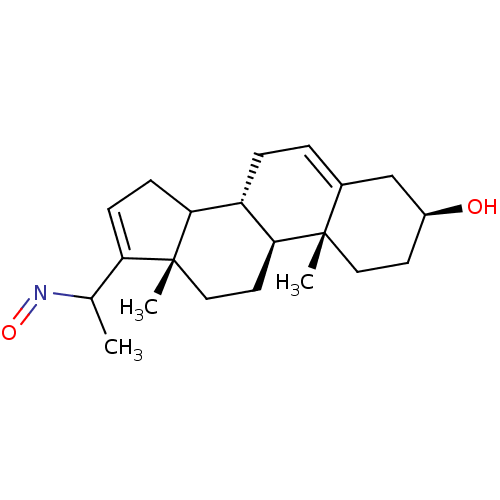
Target3-oxo-5-alpha-steroid 4-dehydrogenase 1(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type I in human DU-145 cell assay at 5 nM of androstenedioneMore data for this Ligand-Target Pair
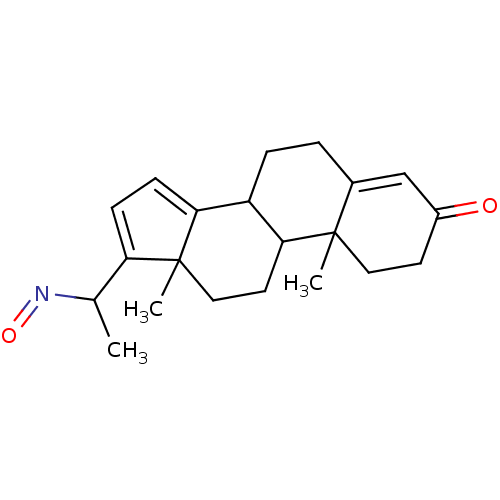
Target3-oxo-5-alpha-steroid 4-dehydrogenase 2(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 60nMAssay Description:Inhibition of Human steroid 5-alpha-reductase type II expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 77nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 110nMpH: 7.4 T: 2°CAssay Description:The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 120nMpH: 7.4 T: 2°CAssay Description:The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Rattus norvegicus (Rat))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 140nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 rat enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 170nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 180nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Rattus norvegicus (Rat))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 180nMAssay Description:Inhibition of rat Cytochrome P450 17More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 230nMAssay Description:Inhibition of Human P450 17 and NADPH-P450 reductase co-expressed in Escherichia coli with 25 uM progesteroneMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 270nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 2(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type 2 from human BPH tissue at 210 nM of testosteroneMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Rattus norvegicus (Rat))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 rat enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 420nMAssay Description:Inhibition of Human P450 17 and NADPH-P450 reductase co-expressed in Escherichia coli with 25 uM progesteroneMore data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 2(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 430nMAssay Description:Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type 2 from human BPH tissue at 210 nM of testosteroneMore data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 2(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 430nMAssay Description:Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type 2 from human BPH tissue at 210 nM of testosteroneMore data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 2(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 470nMAssay Description:Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type 2 from human BPH tissue at 210 nM of testosteroneMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 490nMpH: 7.4 T: 2°CAssay Description:The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 520nMAssay Description:Inhibition of Human P450 17 and NADPH-P450 reductase co-expressed in Escherichia coli with 25 uM progesteroneMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 520nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Rattus norvegicus (Rat))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 520nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 rat enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 1(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 540nMAssay Description:Inhibition of Human steroid 5-alpha-reductase type I expressed in HEK293 cellsMore data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 2(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 580nMAssay Description:Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type 2 from human BPH tissue at 210 nM of testosteroneMore data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 2(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 580nMAssay Description:Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type 2 from human BPH tissue at 210 nM of testosteroneMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 740nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 2(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 860nMAssay Description:Inhibition of Human steroid 5-alpha-reductase type II expressed in HEK293 cellsMore data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 2(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 890nMAssay Description:Inhibition of Human steroid 5-alpha-reductase type II expressed in HEK293 cellsMore data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 1(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 900nMAssay Description:Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type I in human DU-145 cell assay at 5 nM of androstenedioneMore data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 1(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 900nMAssay Description:Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type I in human DU-145 cell assay at 5 nM of androstenedioneMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 1.10E+3nMpH: 7.4 T: 2°CAssay Description:The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 1.18E+3nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+3nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea...More data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+3nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea...More data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 1(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 1.63E+3nMAssay Description:Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type I in human DU-145 cell assay at 5 nM of androstenedioneMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 1.65E+3nMAssay Description:Inhibition of Cytochrome P450 17 of human testicular microsomes at 25 uM progesteroneMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea...More data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 1(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 1.77E+3nMAssay Description:Inhibition of Human steroid 5-alpha-reductase type I expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+3nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea...More data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 1(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 1.95E+3nMAssay Description:Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type I in human DU-145 cell assay at 5 nM of androstenedioneMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+3nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea...More data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+3nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea...More data for this Ligand-Target Pair
Affinity DataIC50: 2.30E+3nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea...More data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 1(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 2.44E+3nMAssay Description:Inhibition of Human steroid 5-alpha-reductase type I expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: >2.50E+3nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair