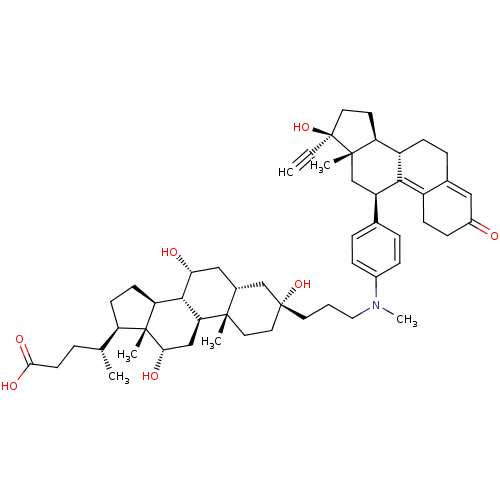
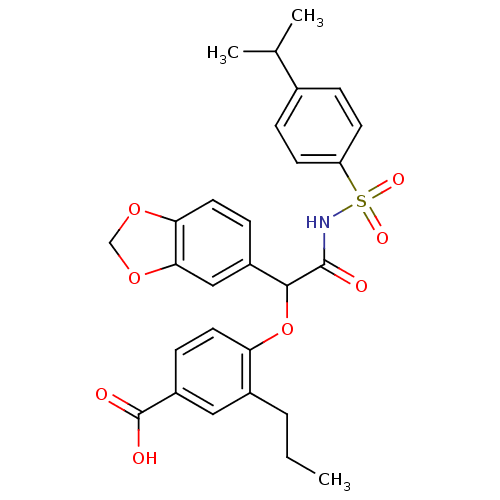
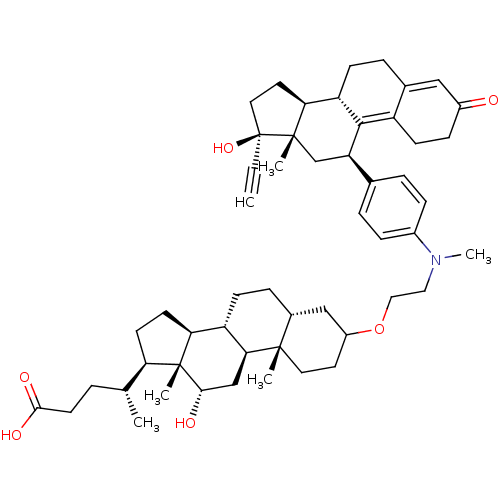
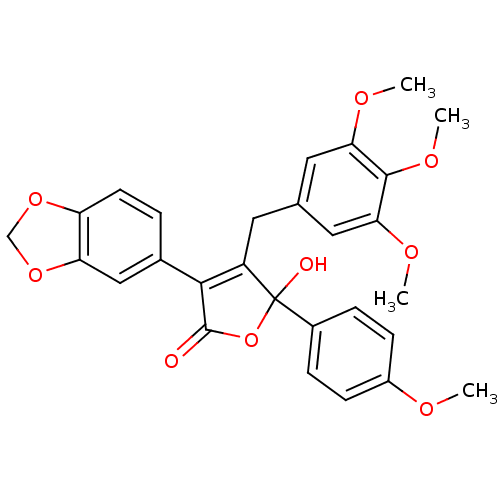
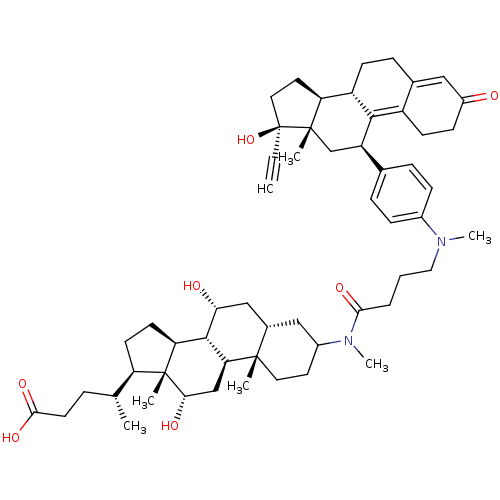
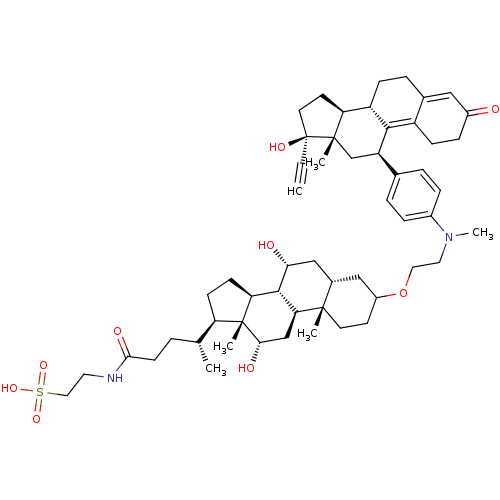
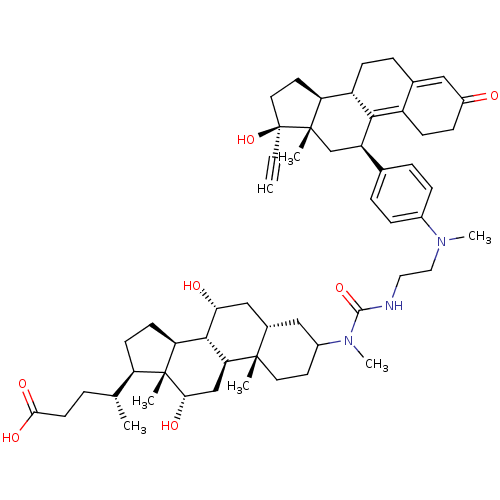
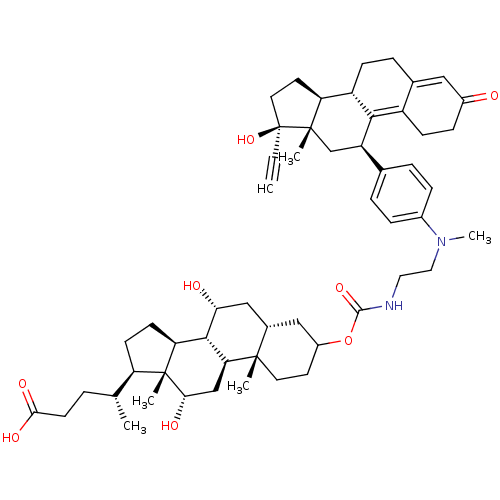
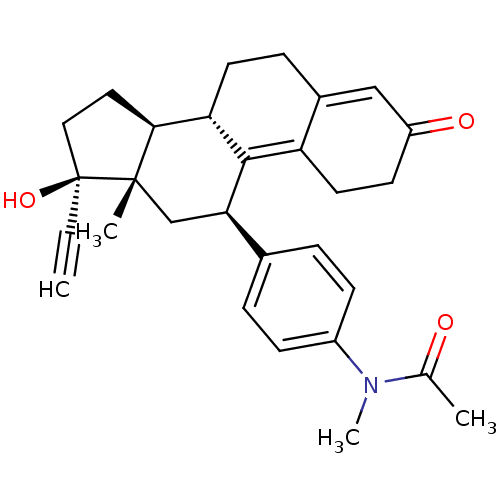
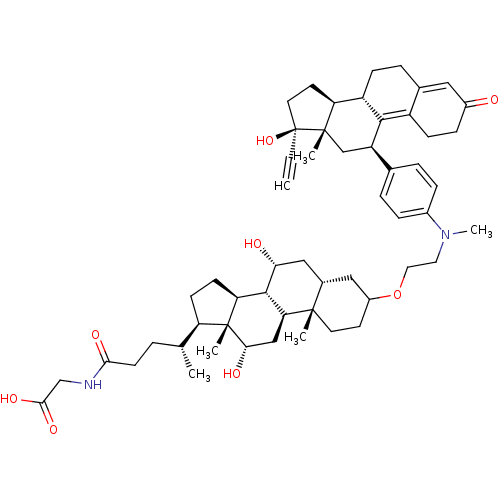
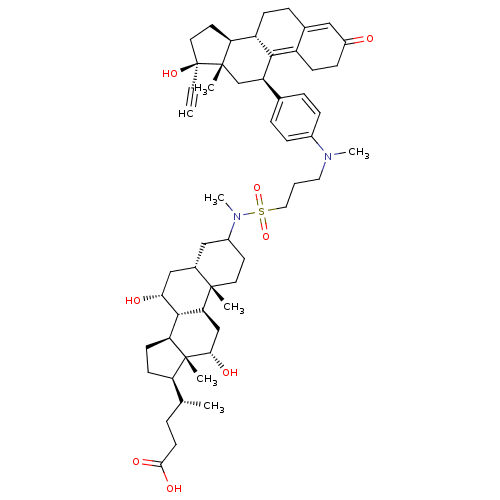
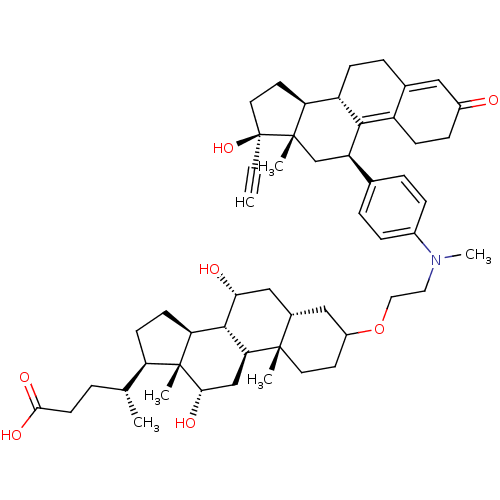
Affinity DataKi: 0.0340nMAssay Description:Inhibitory activity of the compound against human Endothelin A receptor in chinese hamster ovary cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.0390nMAssay Description:Binding affinity against human Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.0470nMAssay Description:binding affinity against human Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.0690nMAssay Description:Inhibitory activity of the compound against human Endothelin A receptor in chinese hamster ovary cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.100nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.110nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.130nMAssay Description:Inhibitory activity of the compound against human Endothelin A receptor in chinese hamster ovary cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.160nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.170nMAssay Description:Inhibitory activity of the compound against human Endothelin A receptor in chinese hamster ovary cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.170nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.180nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Ability of the compound to displace endothelin ([125I]-ET-1) from human Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.210nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.220nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.220nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.270nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.270nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.380nMAssay Description:Inhibition of glucocorticoid receptor dependent alkaline phosphatase activityMore data for this Ligand-Target Pair
Affinity DataKi: 0.390nMAssay Description:Inhibition of human progesterone receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.390nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.420nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.420nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.430nMAssay Description:Inhibitory activity of the compound against human Endothelin A receptor in chinese hamster ovary cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.440nMAssay Description:Inhibition of glucocorticoid receptor dependent alkaline phosphatase activityMore data for this Ligand-Target Pair
Affinity DataKi: 0.460nMAssay Description:Binding affinity against endothelin A receptor in MMQ cells in ratMore data for this Ligand-Target Pair
Affinity DataKi: 0.480nMAssay Description:Inhibition of human progesterone receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.530nMAssay Description:Inhibition of human progesterone receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.640nMAssay Description:Inhibition of human progesterone receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.650nMAssay Description:Inhibition of human androgen receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.670nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.670nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.760nMAssay Description:Inhibition of glucocorticoid receptor dependent alkaline phosphatase activityMore data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.960nMAssay Description:Inhibition of human progesterone receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Inhibition of human progesterone receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Inhibition of glucocorticoid receptor dependent alkaline phosphatase activityMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Inhibition of glucocorticoid receptor dependent alkaline phosphatase activityMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Inhibition of human progesterone receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Ability of the compound to displace endothelin ([125I]-ET-1) from human Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Inhibition of human progesterone receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Inhibition of glucocorticoid receptor dependent alkaline phosphatase activityMore data for this Ligand-Target Pair
Affinity DataKi: 1.40nMAssay Description:Inhibition of glucocorticoid receptor dependent alkaline phosphatase activityMore data for this Ligand-Target Pair
Affinity DataKi: 1.40nMAssay Description:Inhibition of glucocorticoid receptor dependent alkaline phosphatase activityMore data for this Ligand-Target Pair
Affinity DataKi: 1.70nMAssay Description:Inhibition of human progesterone receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.90nMAssay Description:Inhibition of human progesterone receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2nM ΔG°: -49.2kJ/molepH: 7.5 T: 2°CAssay Description:The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas...More data for this Ligand-Target Pair



 3D Structure (crystal)
3D Structure (crystal)