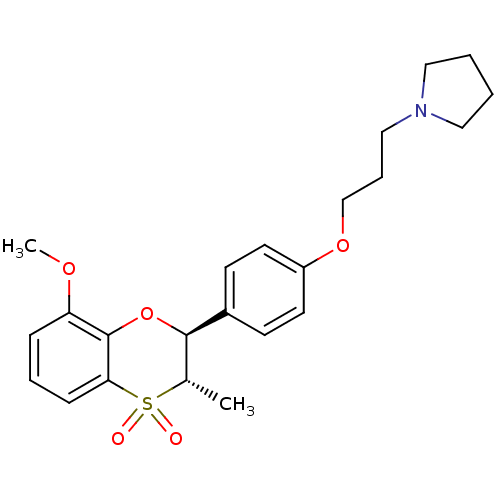
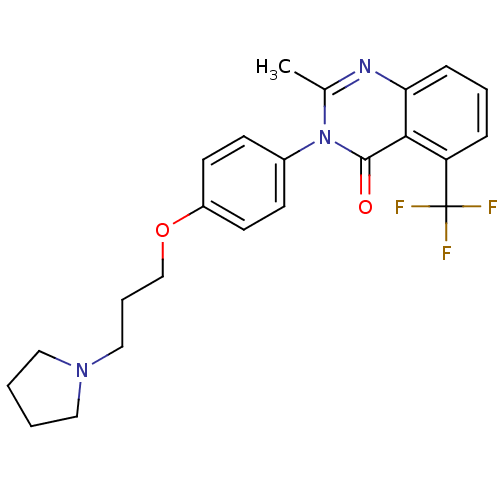
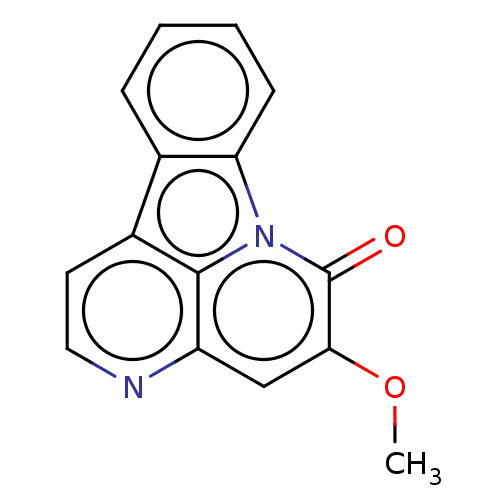
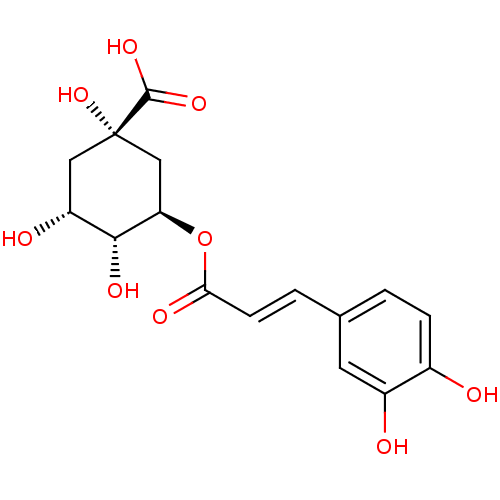
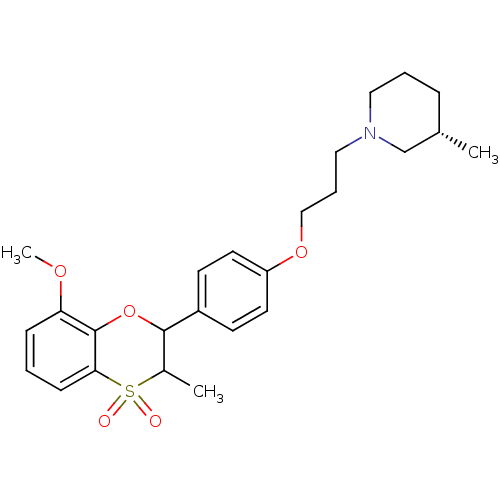
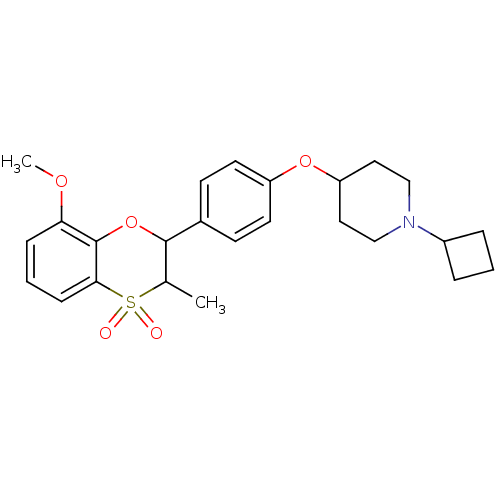
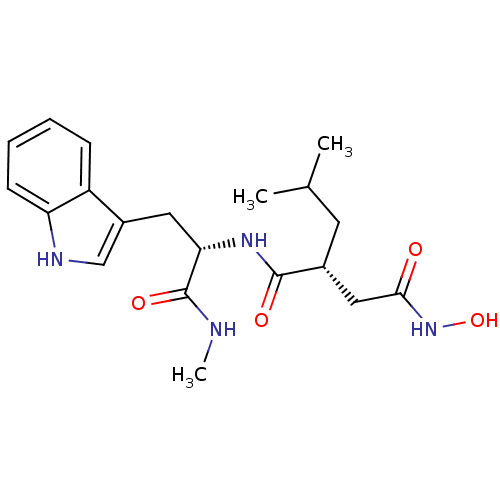
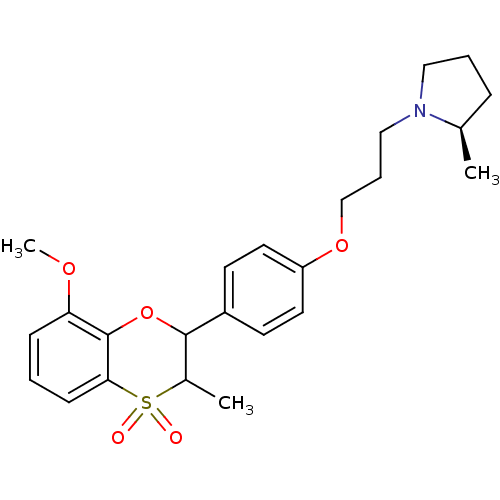
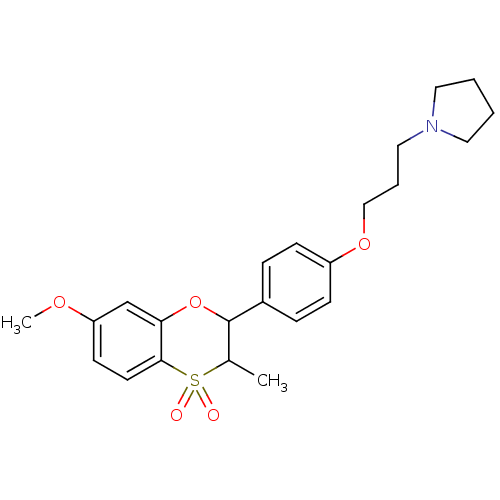
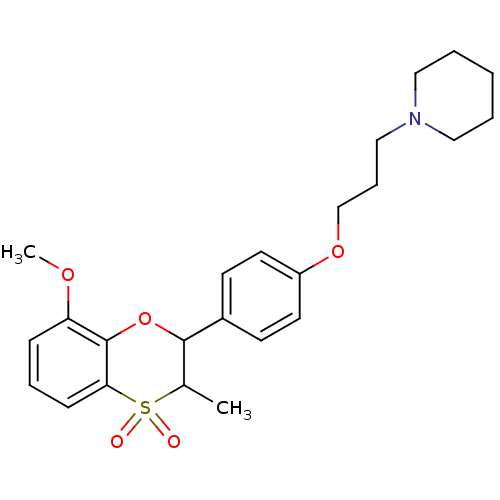
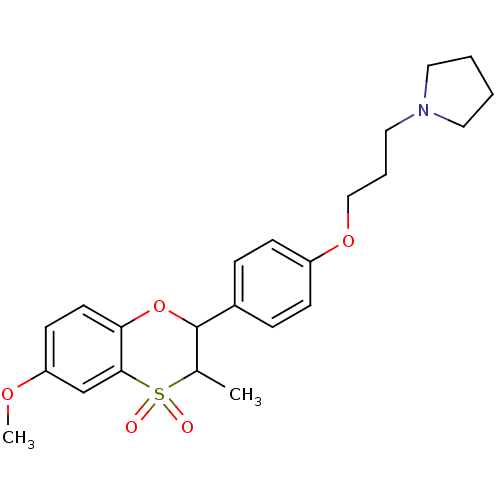
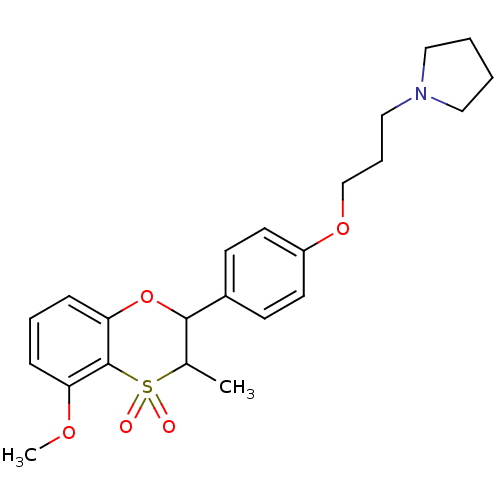
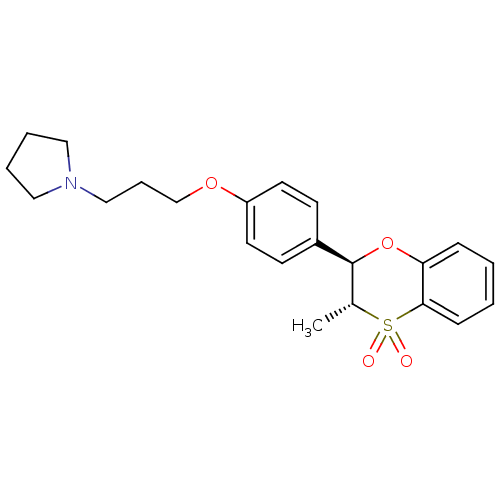
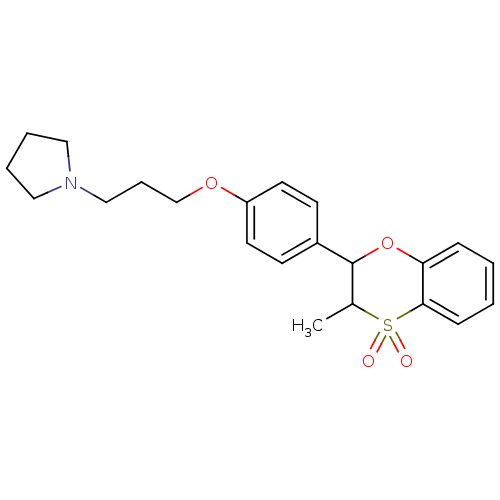
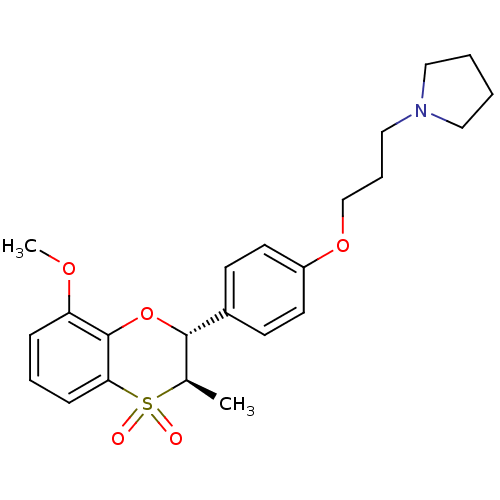
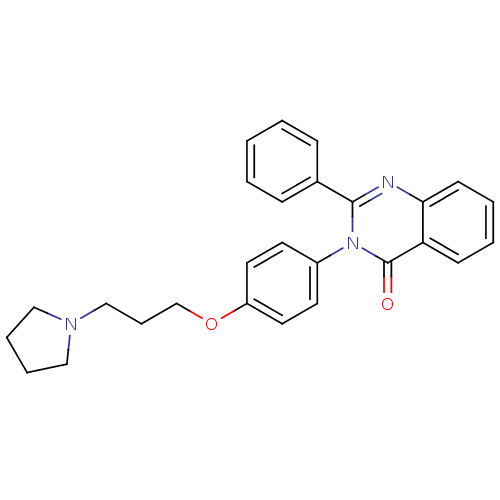
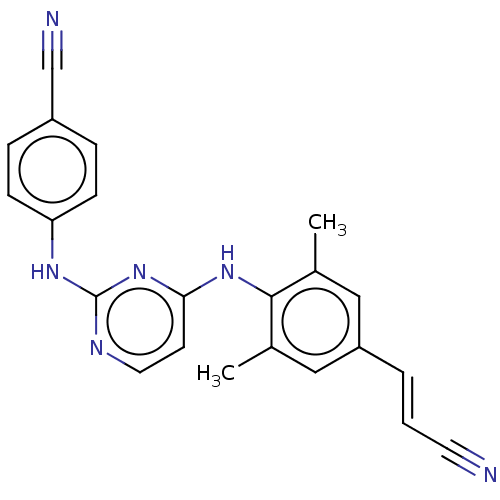
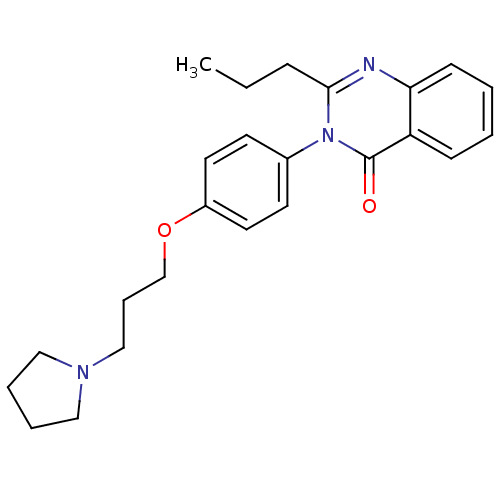
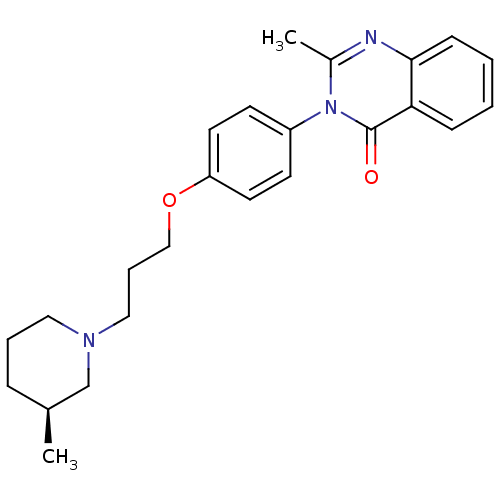
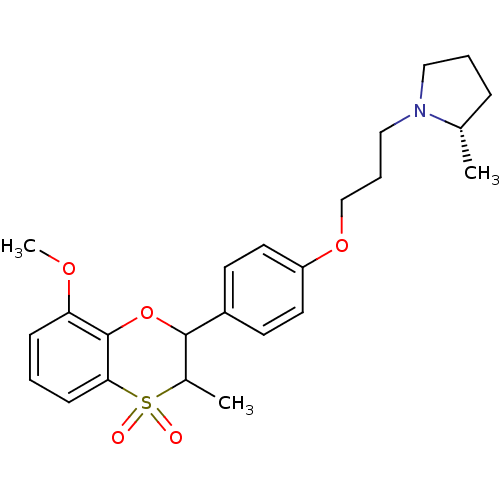
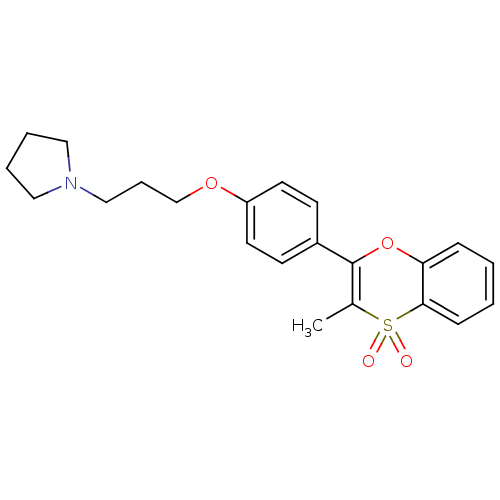
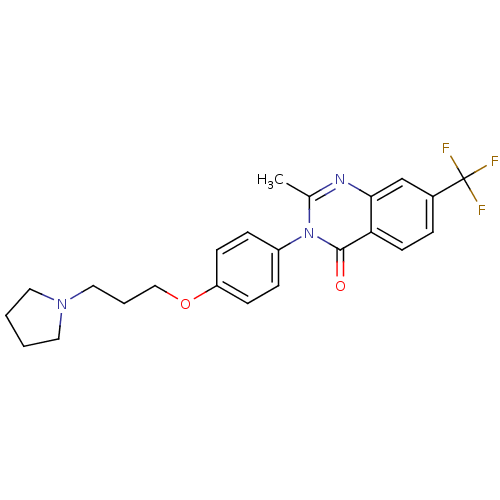
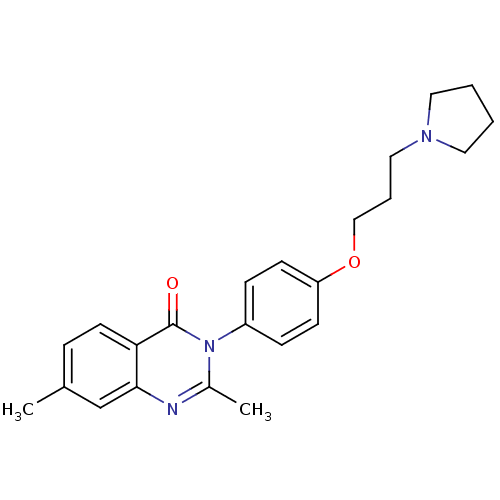
Affinity DataKi: 1nMAssay Description:Displacement of [3H]R-alpha-methylistamine from human histamine H3 receptor by cell-based assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.80nMAssay Description:Displacement of [3H]R-alpha-methylistamine from rat histamine H3 receptor by cell-based assayMore data for this Ligand-Target Pair
TargetHistamine receptor H3(Macaca mulatta (Rhesus macaque))
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataKi: 4.30nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from rhesus monkey histamine H3 receptor expressed in HEK293T cellsMore data for this Ligand-Target Pair
Affinity DataKi: 5.90nMAssay Description:Displacement of [3H]R-alpha-methylistamine from mouse histamine H3 receptor by cell-based assayMore data for this Ligand-Target Pair
Affinity DataKi: 6.80nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 33nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from rat histamine H3 receptor expressed in HEK293 cells coexpressed with CRE-beta-lactamaseMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Toho University
Curated by ChEMBL
Toho University
Curated by ChEMBL
Affinity DataKi: 7.07E+3nMAssay Description:Competitive inhibition of PTP1B (unknown origin) assessed as hydrolysis of para-nitrophenylphosphate by Lineweaver-Burk plotMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Toho University
Curated by ChEMBL
Toho University
Curated by ChEMBL
Affinity DataKi: 1.03E+4nMAssay Description:Non-competitive inhibition of PTP1B (unknown origin) assessed as hydrolysis of para-nitrophenylphosphate by Lineweaver-Burk plotMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Toho University
Curated by ChEMBL
Toho University
Curated by ChEMBL
Affinity DataKi: 1.29E+4nMAssay Description:Non-competitive inhibition of PTP1B (unknown origin) assessed as hydrolysis of para-nitrophenylphosphate by Lineweaver-Burk plotMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Toho University
Curated by ChEMBL
Toho University
Curated by ChEMBL
Affinity DataKi: 1.51E+4nMAssay Description:Non-competitive inhibition of PTP1B (unknown origin) using p-NPP as substrate by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Toho University
Curated by ChEMBL
Toho University
Curated by ChEMBL
Affinity DataKi: 1.84E+4nMAssay Description:Non-competitive inhibition of PTP1B (unknown origin) assessed as hydrolysis of para-nitrophenylphosphate by Lineweaver-Burk plotMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Toho University
Curated by ChEMBL
Toho University
Curated by ChEMBL
Affinity DataKi: 1.99E+4nMAssay Description:Non-competitive inhibition of PTP1B (unknown origin) assessed as hydrolysis of para-nitrophenylphosphate by Lineweaver-Burk plotMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Toho University
Curated by ChEMBL
Toho University
Curated by ChEMBL
Affinity DataKi: 2.01E+4nMAssay Description:Non-competitive inhibition of PTP1B (unknown origin) assessed as hydrolysis of para-nitrophenylphosphate by Lineweaver-Burk plotMore data for this Ligand-Target Pair
Affinity DataIC50: 0.00200nMAssay Description:Inhibition of human activated TAFI using Hip-Arg as substrate incubated for 10 mins prior to substrate addition measured after 30 mins by spectrophot...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0250nMAssay Description:Inhibition of human activated TAFI using Hip-Arg as substrate incubated for 10 mins prior to substrate addition measured after 30 mins by spectrophot...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Mitsubishi Tanabe Pharma
Curated by ChEMBL
Mitsubishi Tanabe Pharma
Curated by ChEMBL
Affinity DataIC50: 0.0610nMAssay Description:Inhibition of human N-terminal FLAG-tagged PDE10A (1 to 90 residues) expressed in using African green monkey COS7 cells using [3H]cAMP or [3H]cGMP as...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Mitsubishi Tanabe Pharma
Curated by ChEMBL
Mitsubishi Tanabe Pharma
Curated by ChEMBL
Affinity DataIC50: 0.0620nMAssay Description:Inhibition of human N-terminal FLAG-tagged PDE10A (1 to 90 residues) expressed in using African green monkey COS7 cells using [3H]cAMP or [3H]cGMP as...More data for this Ligand-Target Pair
Affinity DataIC50: 0.160nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel...More data for this Ligand-Target Pair
Affinity DataIC50: 0.160nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel...More data for this Ligand-Target Pair
Affinity DataIC50: 0.177nMAssay Description:Inhibition of human MMP-9 by fluorometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.190nMAssay Description:Inhibition of pig pancreatic carboxypeptidase B using Hip-Arg as substrate incubated for 10 mins prior to substrate addition measured after 30 mins b...More data for this Ligand-Target Pair
Affinity DataIC50: 0.210nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel...More data for this Ligand-Target Pair
Affinity DataIC50: 0.320nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel...More data for this Ligand-Target Pair
Affinity DataIC50: 0.340nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel...More data for this Ligand-Target Pair
Affinity DataIC50: 0.390nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel...More data for this Ligand-Target Pair
Affinity DataIC50: 0.422nMAssay Description:Inhibition of human MMP-2 by fluorometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.430nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel...More data for this Ligand-Target Pair
Affinity DataIC50: 0.440nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel...More data for this Ligand-Target Pair
Affinity DataIC50: 0.607nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel...More data for this Ligand-Target Pair
Affinity DataIC50: 0.660nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.680nMAssay Description:Inhibition of recombinant HIV1 reverse transcriptase p66/p51 Y181C mutant expressed in Escherichia coli BL21 (DE3) pLysS cells preincubated followed ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.690nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.720nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.810nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.820nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel...More data for this Ligand-Target Pair
Affinity DataIC50: 0.840nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel...More data for this Ligand-Target Pair
Affinity DataIC50: 0.960nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.980nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMT: 2°CAssay Description:The frozen cell membrane fraction prepared as described above was thawed before use, and the resultant was diluted with a buffer for a binding assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Inhibition of pig pancreatic carboxypeptidase B using Hip-Arg as substrate incubated for 10 mins prior to substrate addition measured after 30 mins b...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Inhibition of human activated TAFI using Hip-Arg as substrate incubated for 10 mins prior to substrate addition measured after 30 mins by spectrophot...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Inhibition of pig pancreatic carboxypeptidase B using Hip-Arg as substrate incubated for 10 mins prior to substrate addition measured after 30 mins b...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair