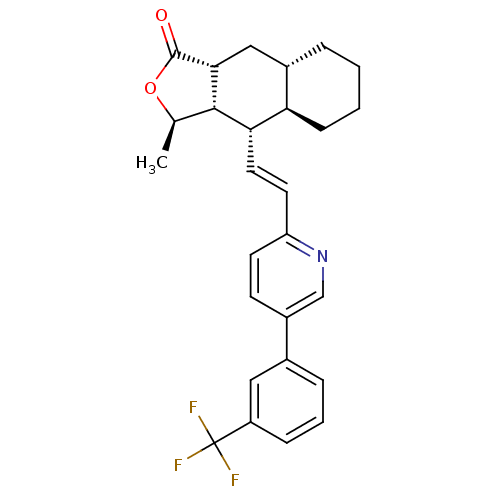
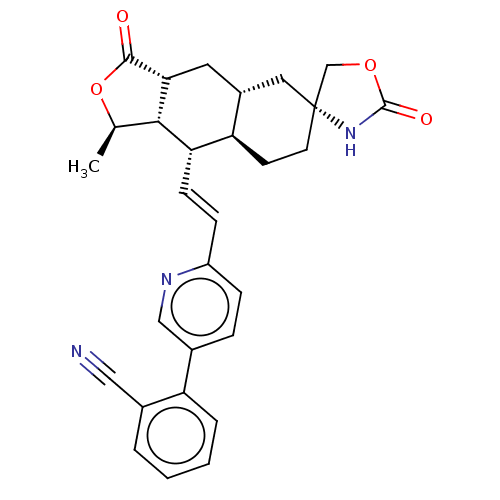
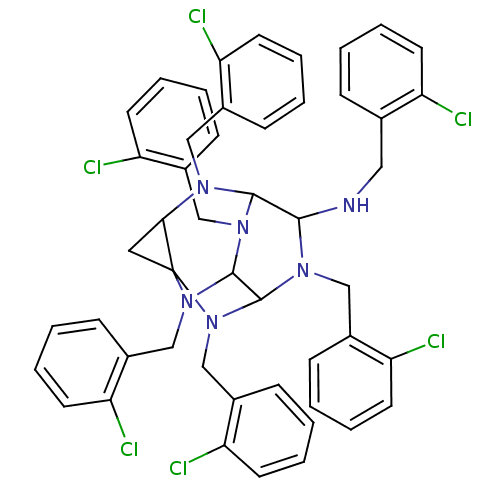
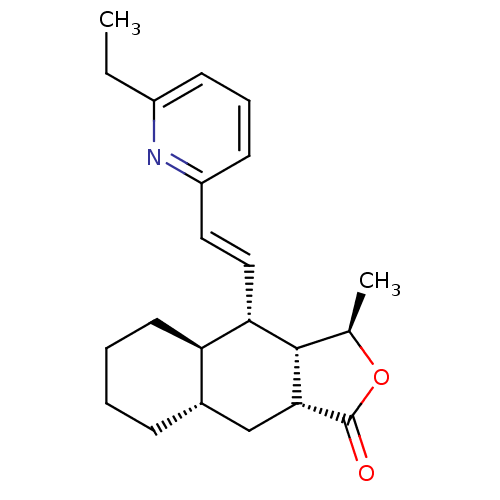
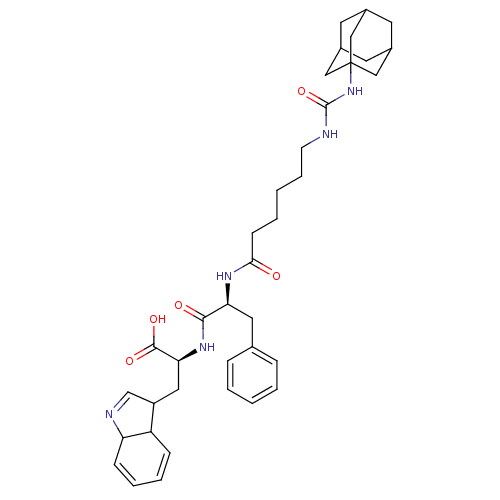
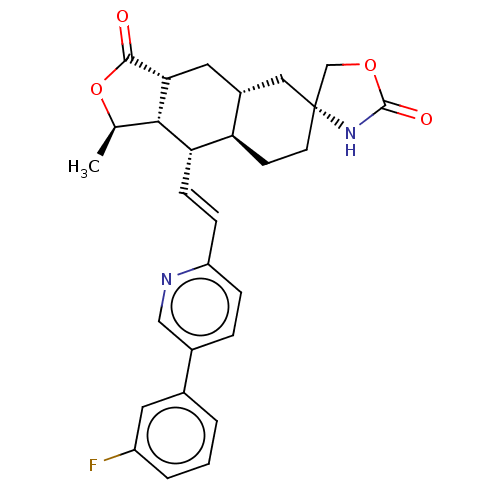
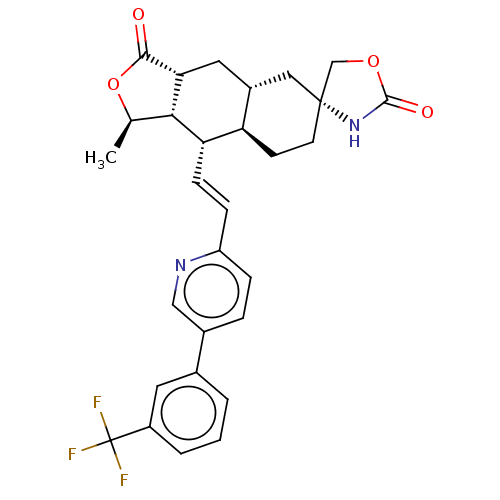
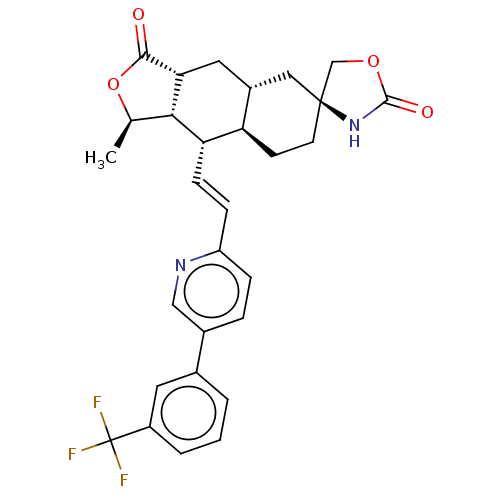
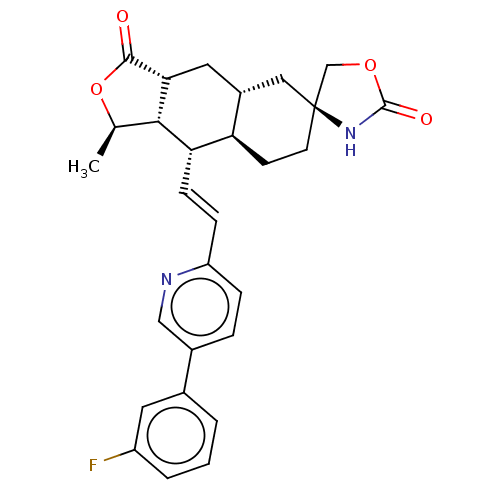
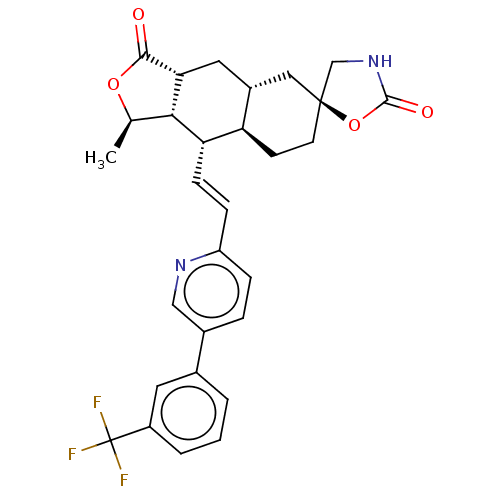
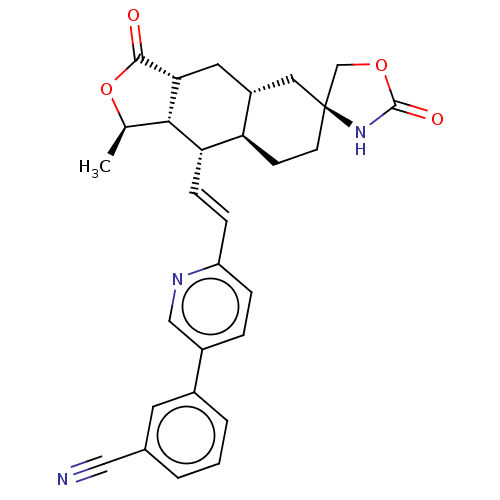
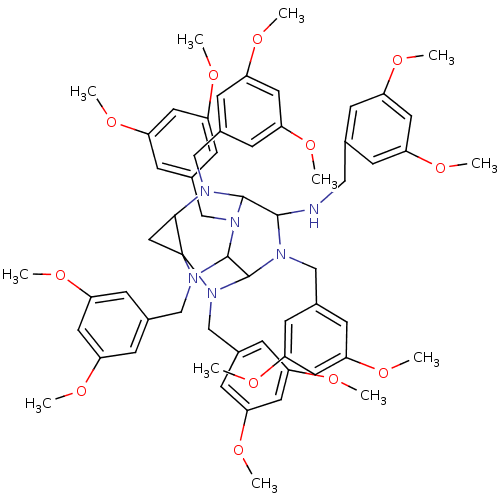
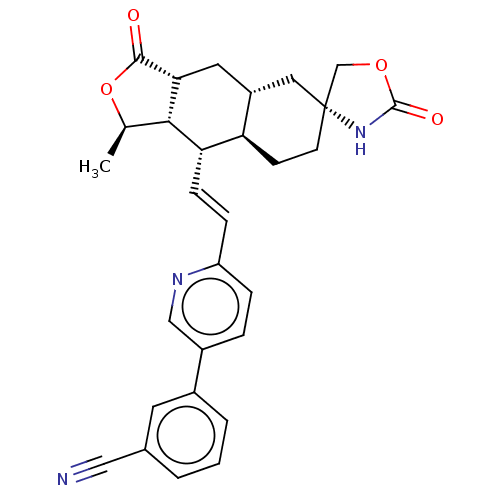
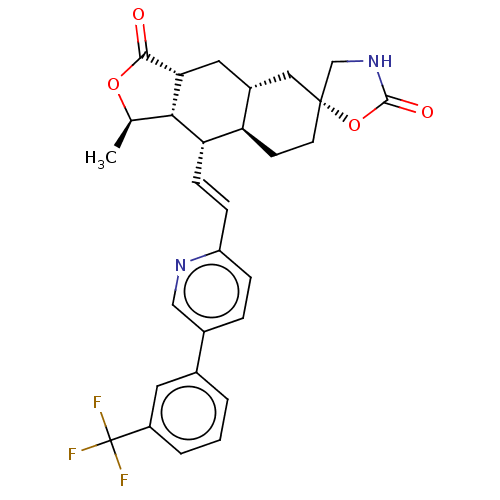
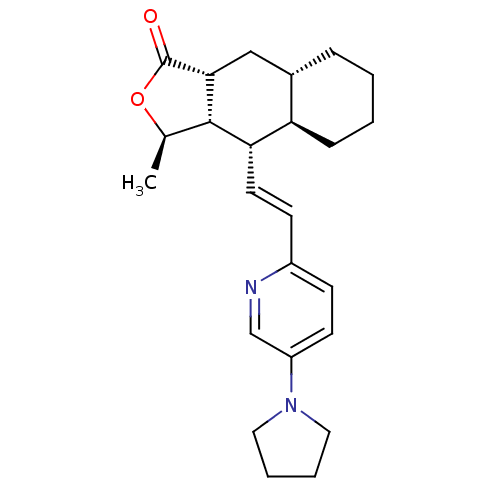
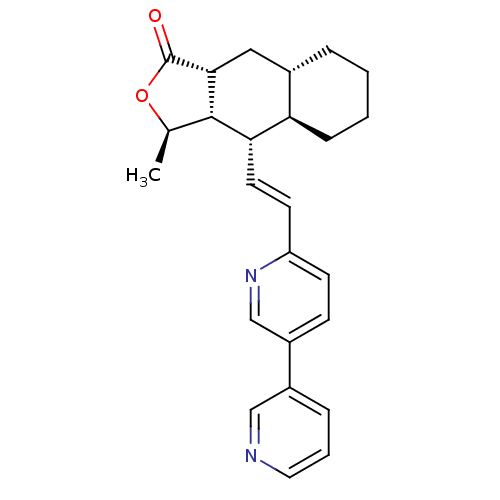
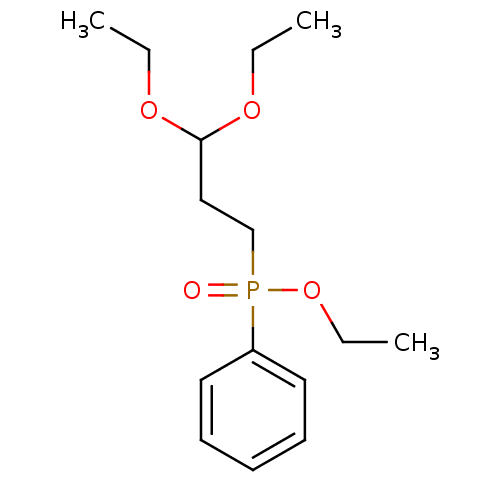
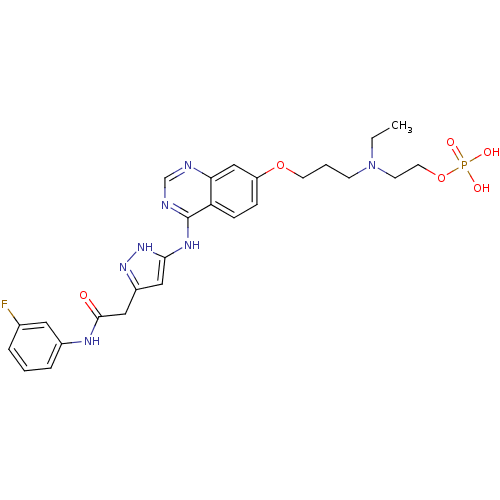
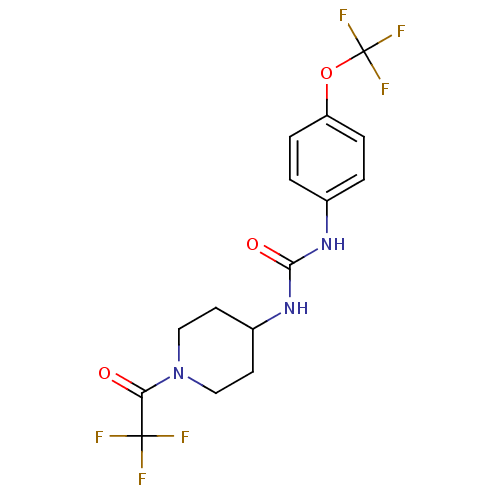
TargetProteinase-activated receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 2.70nMAssay Description:Inhibitory constant aganist Protease-activated receptor 1More data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 5.10nMAssay Description:Displacement of [3H]haTRAP from PAR-1 receptor in human platelet membranes after 1 hr by scintillation counting analysisMore data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 8.90nMAssay Description:Displacement of [3H]haTRAP from PAR-1 receptor in human platelet membranes after 1 hr by scintillation counting analysisMore data for this Ligand-Target Pair
TargetCarboxylic ester hydrolase(Equus caballus (Horse))
National Chung-Hsing University
Curated by ChEMBL
National Chung-Hsing University
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Inhibitory activity of the compound against ButyrylcholinesteraseMore data for this Ligand-Target Pair
TargetCarboxylic ester hydrolase(Equus caballus (Horse))
National Chung-Hsing University
Curated by ChEMBL
National Chung-Hsing University
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Inhibitory activity of the compound against ButyrylcholinesteraseMore data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Displacement of [3H]haTRAP from PAR1 in human platelet membraneMore data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 12nMAssay Description:Inhibitory constant aganist Protease-activated receptor 1More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California
Curated by ChEMBL
University Of California
Curated by ChEMBL
Affinity DataKi: 15nMAssay Description:Inhibition of human recombinant soluble Epoxide hydrolaseMore data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 16nMAssay Description:Displacement of [3H]haTRAP from PAR-1 receptor in human platelet membranes after 1 hr by scintillation counting analysisMore data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 21nMAssay Description:Displacement of [3H]haTRAP from PAR-1 receptor in human platelet membranes after 1 hr by scintillation counting analysisMore data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 25nMAssay Description:Displacement of [3H]haTRAP from PAR-1 receptor in human platelet membranes after 1 hr by scintillation counting analysisMore data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 27nMAssay Description:Displacement of [3H]haTRAP from PAR-1 receptor in human platelet membranes after 1 hr by scintillation counting analysisMore data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 28nMAssay Description:Displacement of [3H]haTRAP from PAR-1 receptor in human platelet membranes after 1 hr by scintillation counting analysisMore data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 32nMAssay Description:Displacement of [3H]haTRAP from PAR-1 receptor in human platelet membranes after 1 hr by scintillation counting analysisMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
National Chung-Hsing University
Curated by ChEMBL
National Chung-Hsing University
Curated by ChEMBL
Affinity DataKi: 39nMAssay Description:Inhibitory activity of the compound against acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
National Chung-Hsing University
Curated by ChEMBL
National Chung-Hsing University
Curated by ChEMBL
Affinity DataKi: 39nMAssay Description:Inhibitory activity of the compound against acetylcholinesteraseMore data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 43nMAssay Description:Displacement of [3H]haTRAP from PAR-1 receptor in human platelet membranes after 1 hr by scintillation counting analysisMore data for this Ligand-Target Pair
TargetCarboxylic ester hydrolase(Equus caballus (Horse))
National Chung-Hsing University
Curated by ChEMBL
National Chung-Hsing University
Curated by ChEMBL
Affinity DataKi: 60nMAssay Description:Inhibitory activity of the compound against ButyrylcholinesteraseMore data for this Ligand-Target Pair
TargetCarboxylic ester hydrolase(Equus caballus (Horse))
National Chung-Hsing University
Curated by ChEMBL
National Chung-Hsing University
Curated by ChEMBL
Affinity DataKi: 60nMAssay Description:Inhibitory activity of the compound against ButyrylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
National Chung-Hsing University
Curated by ChEMBL
National Chung-Hsing University
Curated by ChEMBL
Affinity DataKi: 130nMAssay Description:Inhibitory activity of the compound against acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
National Chung-Hsing University
Curated by ChEMBL
National Chung-Hsing University
Curated by ChEMBL
Affinity DataKi: 130nMAssay Description:Inhibitory activity of the compound against acetylcholinesteraseMore data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 232nMAssay Description:Displacement of [3H]haTRAP from PAR-1 receptor in human platelet membranes after 1 hr by scintillation counting analysisMore data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 267nMAssay Description:Displacement of [3H]haTRAP from PAR-1 receptor in human platelet membranes after 1 hr by scintillation counting analysisMore data for this Ligand-Target Pair
TargetCarboxylic ester hydrolase(Equus caballus (Horse))
National Chung-Hsing University
Curated by ChEMBL
National Chung-Hsing University
Curated by ChEMBL
Affinity DataKi: 310nMAssay Description:Inhibitory activity of the compound against ButyrylcholinesteraseMore data for this Ligand-Target Pair
TargetCarboxylic ester hydrolase(Equus caballus (Horse))
National Chung-Hsing University
Curated by ChEMBL
National Chung-Hsing University
Curated by ChEMBL
Affinity DataKi: 310nMAssay Description:Inhibitory activity of the compound against ButyrylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
National Chung-Hsing University
Curated by ChEMBL
National Chung-Hsing University
Curated by ChEMBL
Affinity DataKi: 320nMAssay Description:Inhibitory activity of the compound against acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
National Chung-Hsing University
Curated by ChEMBL
National Chung-Hsing University
Curated by ChEMBL
Affinity DataKi: 320nMAssay Description:Inhibitory activity of the compound against acetylcholinesteraseMore data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 392nMAssay Description:Displacement of [3H]haTRAP from PAR-1 receptor in human platelet membranes after 1 hr by scintillation counting analysisMore data for this Ligand-Target Pair
TargetCannabinoid receptor 2(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 487nMAssay Description:Binding affinity to cannabinoid CB2 receptorMore data for this Ligand-Target Pair
TargetCarboxylic ester hydrolase(Equus caballus (Horse))
National Chung-Hsing University
Curated by ChEMBL
National Chung-Hsing University
Curated by ChEMBL
Affinity DataKi: 900nMAssay Description:Inhibitory activity of the compound against ButyrylcholinesteraseMore data for this Ligand-Target Pair
TargetCarboxylic ester hydrolase(Equus caballus (Horse))
National Chung-Hsing University
Curated by ChEMBL
National Chung-Hsing University
Curated by ChEMBL
Affinity DataKi: 900nMAssay Description:Inhibitory activity of the compound against ButyrylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
National Chung-Hsing University
Curated by ChEMBL
National Chung-Hsing University
Curated by ChEMBL
Affinity DataKi: 4.00E+3nMAssay Description:Inhibitory activity of the compound against acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
National Chung-Hsing University
Curated by ChEMBL
National Chung-Hsing University
Curated by ChEMBL
Affinity DataKi: 4.00E+3nMAssay Description:Inhibitory activity of the compound against acetylcholinesteraseMore data for this Ligand-Target Pair
TargetCannabinoid receptor 2(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 1.30E+4nMAssay Description:Binding affinity to cannabinoid CB2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 3.00E+4nMAssay Description:Tested for its ability to inhibit rabbit liver aldehyde oxidase catalyzed oxidation of N-methyl-nicotinamide (NMN)More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+5nMAssay Description:Tested for its ability to inhibit rabbit liver aldehyde oxidase catalyzed oxidation of N-methyl-nicotinamide (NMN)More data for this Ligand-Target Pair
Affinity DataKi: 2.00E+5nMAssay Description:Tested for its ability to inhibit rabbit liver aldehyde oxidase catalyzed oxidation of N-methyl-nicotinamide (NMN)More data for this Ligand-Target Pair
Affinity DataKi: 2.00E+5nMAssay Description:Tested for its ability to inhibit rabbit liver aldehyde oxidase catalyzed oxidation of N-methyl-nicotinamide (NMN)More data for this Ligand-Target Pair
Affinity DataKi: 3.00E+5nMAssay Description:Tested for its ability to inhibit rabbit liver aldehyde oxidase catalyzed oxidation of N-methyl-nicotinamide (NMN)More data for this Ligand-Target Pair
Affinity DataKi: 3.00E+5nMAssay Description:Tested for its ability to inhibit rabbit liver aldehyde oxidase catalyzed oxidation of N-methyl-nicotinamide (NMN)More data for this Ligand-Target Pair
Affinity DataKi: 4.00E+5nMAssay Description:Tested for its ability to inhibit rabbit liver aldehyde oxidase catalyzed oxidation of N-methyl-nicotinamide (NMN)More data for this Ligand-Target Pair
Affinity DataKi: 1.50E+6nMAssay Description:Tested for its ability to inhibit rabbit liver aldehyde oxidase catalyzed oxidation of N-methyl-nicotinamide (NMN)More data for this Ligand-Target Pair
TargetMast/stem cell growth factor receptor Kit(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 0.0200nMAssay Description:Inhibition of human c-KIT A loop exon 17 D820Y single mutant using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma-ATP by hotspot kinase ass...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:Inhibition of wild type FLT3 (unknown origin) expressed in HEK293T cells assessed as decrease in FLT3 phosphorylation at 0.1 to 1000 nM after 1 hr by...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:Inhibition of wild type FLT3 ITD mutant (unknown origin) expressed in HEK293T cells assessed as decrease in FLT3 ITD phosphorylation at 0.1 to 1000 n...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:Inhibition of wild type FLT3 D835Y mutant (unknown origin) expressed in HEK293T cells assessed as decrease in FLT3 D835Y phosphorylation at 0.1 to 10...More data for this Ligand-Target Pair
TargetMast/stem cell growth factor receptor Kit(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 0.120nMAssay Description:Inhibition of human c-KIT A loop exon 17 Y823D single mutant using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma-ATP by hotspot kinase ass...More data for this Ligand-Target Pair
TargetMast/stem cell growth factor receptor Kit(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 0.180nMAssay Description:Inhibition of human c-KIT A loop exon 11/17 V560G/N822K double mutant using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma-ATP by hotspot k...More data for this Ligand-Target Pair
TargetAurora kinase A(Homo sapiens (Human))
Taiwan National Health Research Institutes
Curated by ChEMBL
Taiwan National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 0.370nMAssay Description:Inhibition of recombinant GST-tagged N-terminal truncated human Aurora A (123 to 401 residues) expressed in Sf9 insect cell using tetra-LRRASLG pepti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Inhibition assay of human soluble epoxide hydrolases.More data for this Ligand-Target Pair