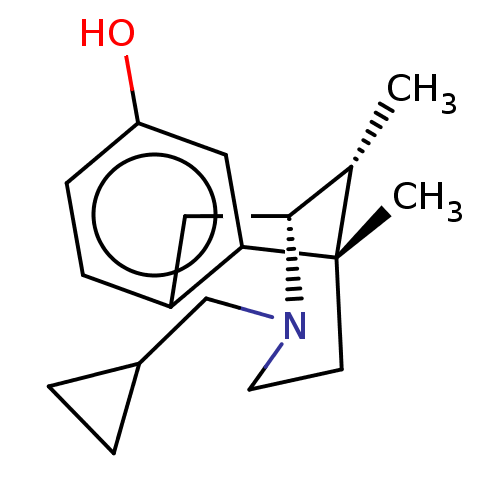
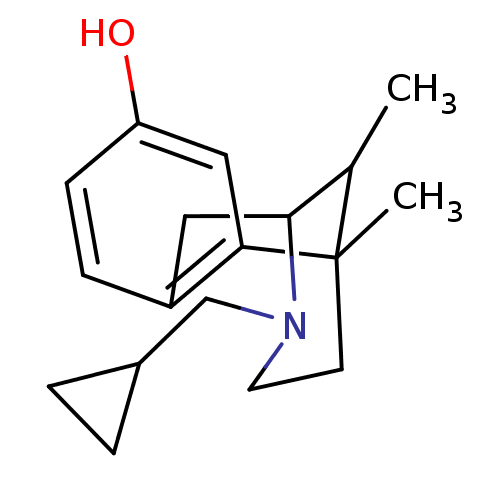
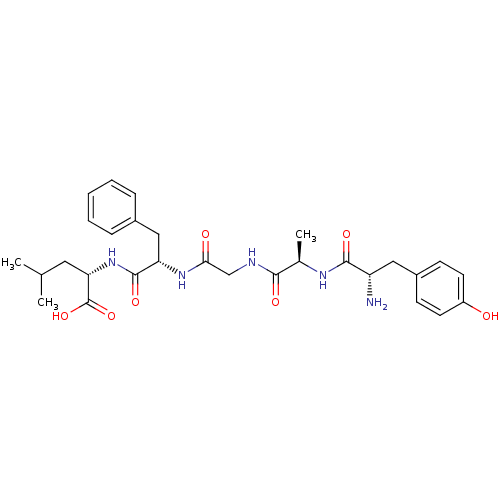
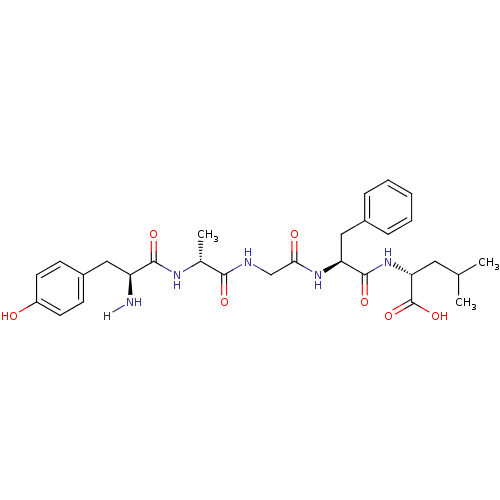
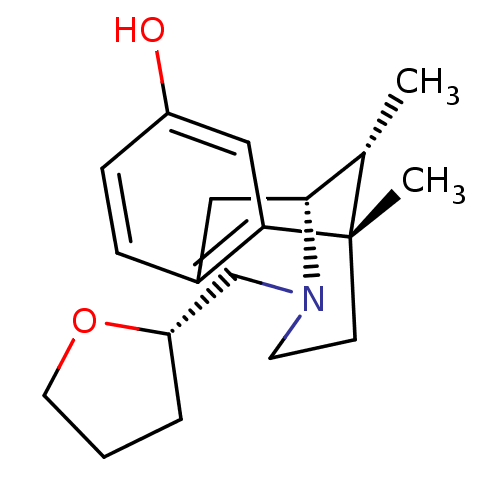
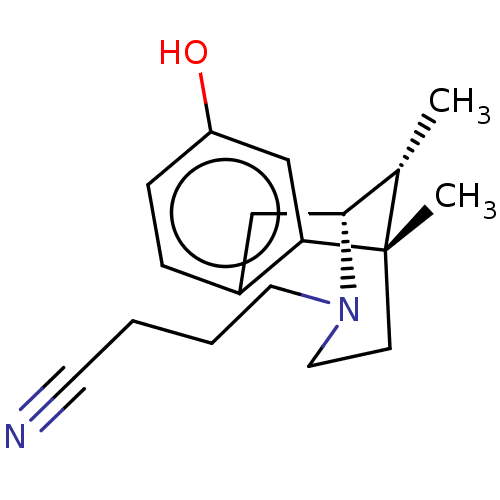
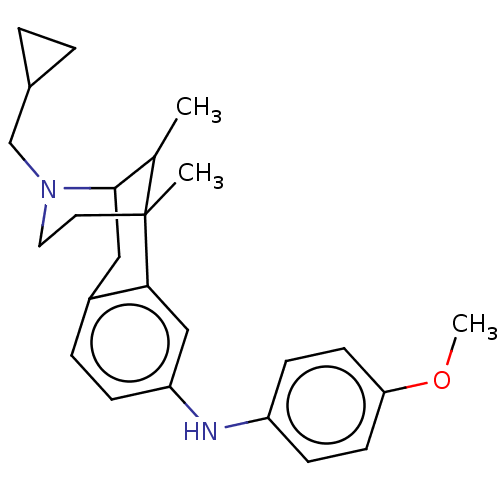
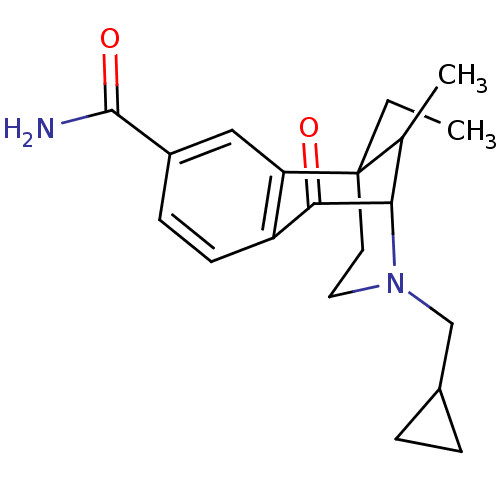
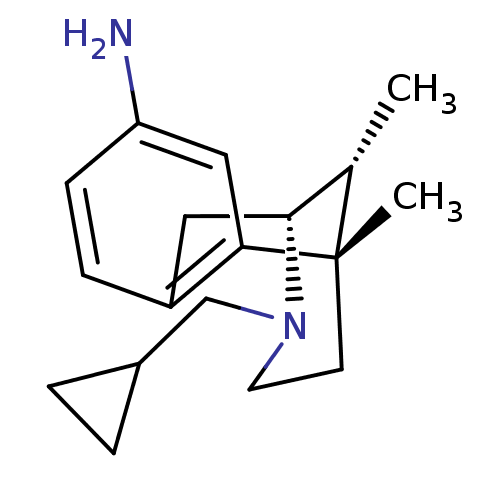
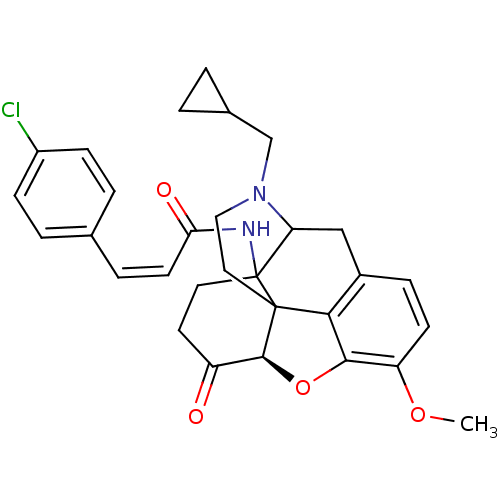
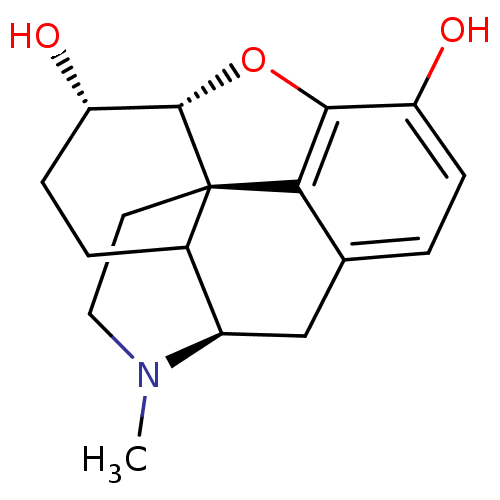
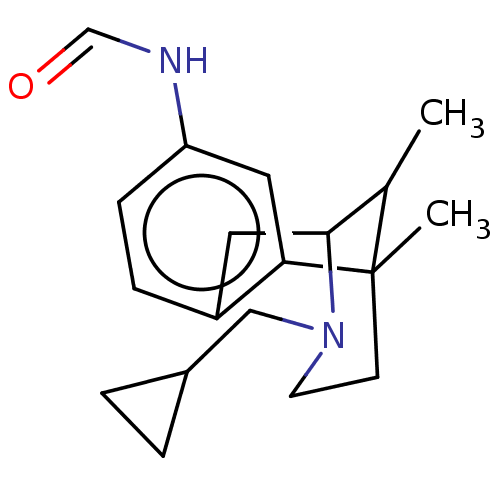
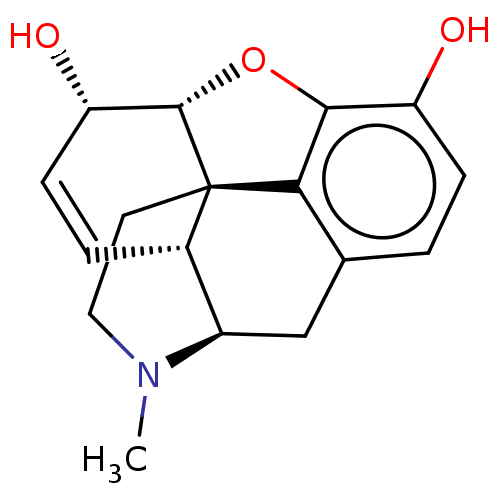
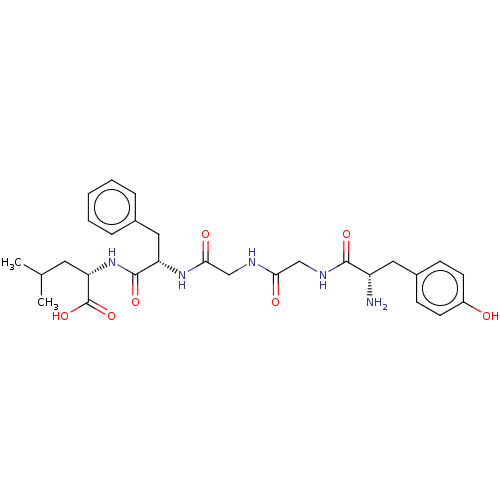
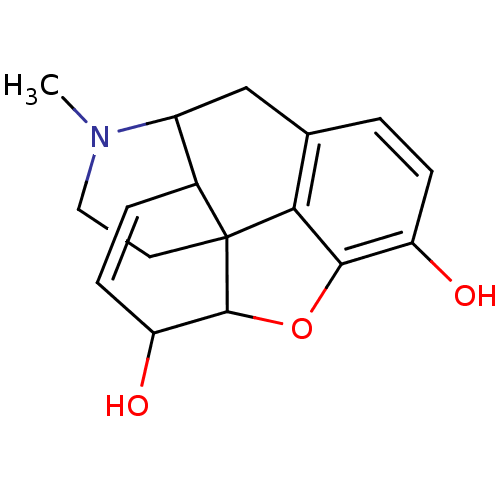
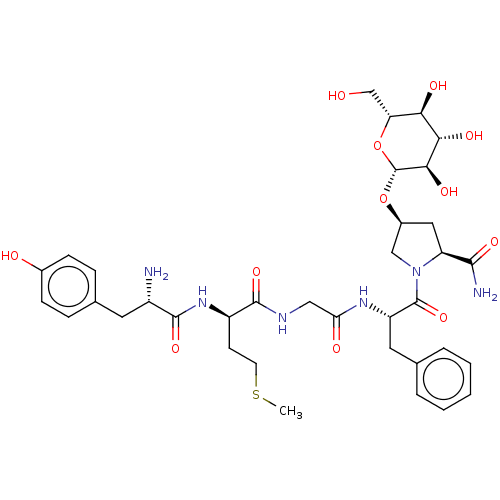
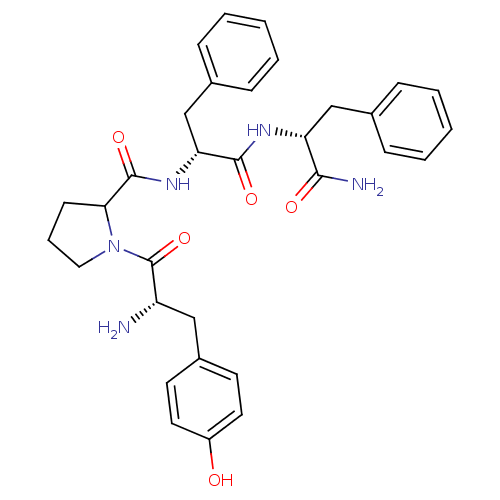
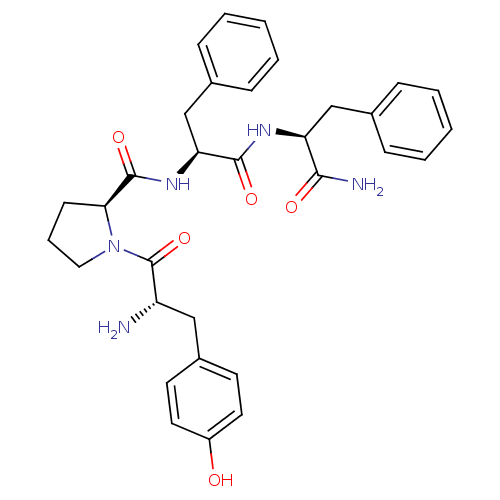
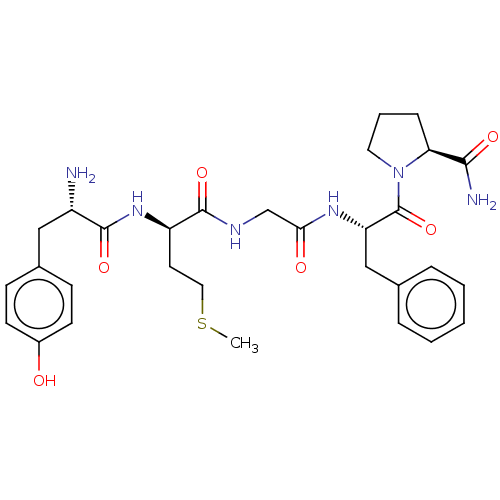
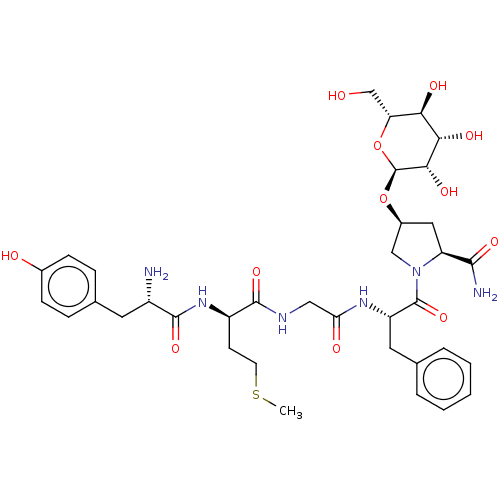
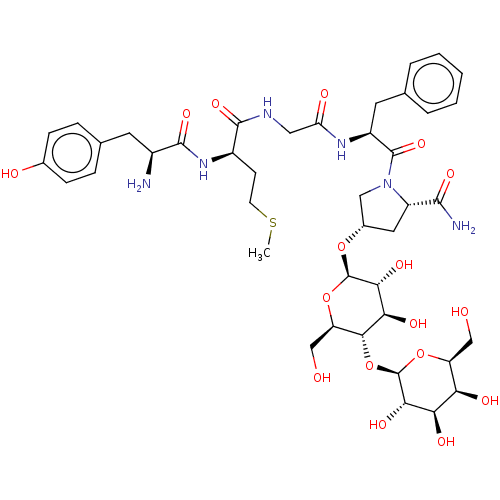
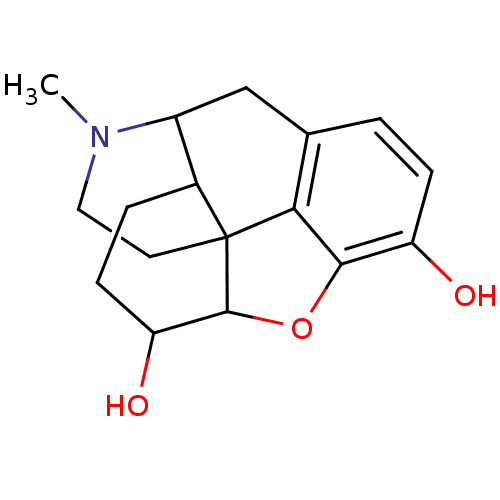
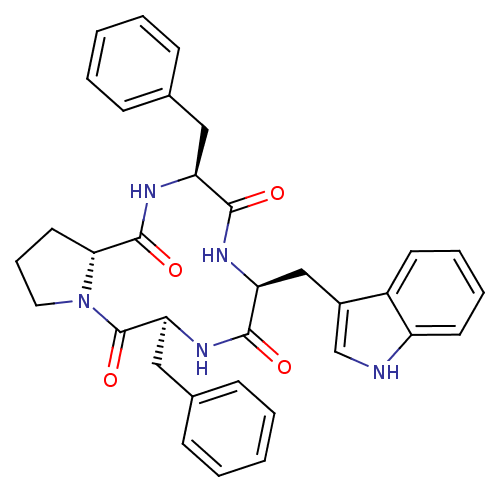
Affinity DataKi: 0.580nMAssay Description:Displacement of [3H]Naltrindole from DOR in guinea pig brain membranes after 3 hrs by scintillation counting methodMore data for this Ligand-Target Pair
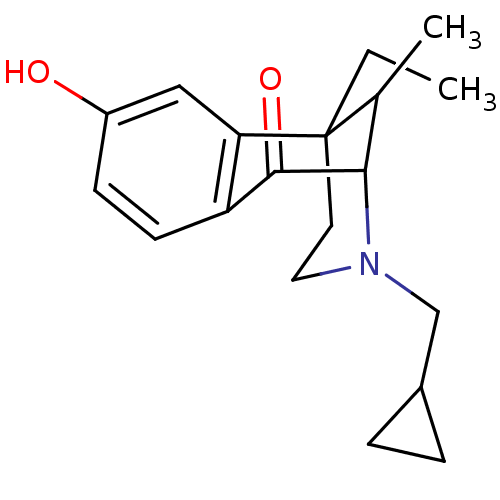
Affinity DataKi: 1.10nMAssay Description:Displacement of [3H]Naltrindole from DOR in guinea pig brain membranes after 3 hrs by scintillation counting methodMore data for this Ligand-Target Pair
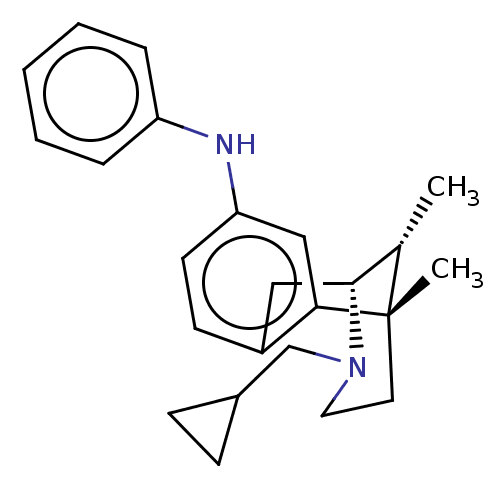
Affinity DataKi: 2.5nMAssay Description:Displacement of [3H]Naltrindole from DOR in guinea pig brain membranes after 3 hrs by scintillation counting methodMore data for this Ligand-Target Pair
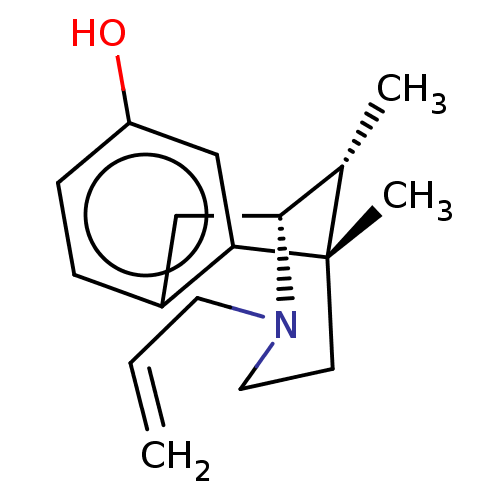
Affinity DataKi: 3.40nMAssay Description:Displacement of [3H]Naltrindole from DOR in guinea pig brain membranes after 3 hrs by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 4.20nMAssay Description:Displacement of [3H]naltrindole from DOR in monkey brain cortex membranesMore data for this Ligand-Target Pair
Affinity DataKi: 5.20nMAssay Description:Displacement of [3H]Naltrindole from DOR in guinea pig brain membranes after 3 hrs by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 5.5nMAssay Description:Displacement of [3H]naltrindole from DOR in monkey brain cortex membranesMore data for this Ligand-Target Pair
Affinity DataKi: 5.80nMAssay Description:Displacement of [3H]naltrindole from DOR in monkey brain cortex membranesMore data for this Ligand-Target Pair
Affinity DataKi: 8.30nMAssay Description:Displacement of [3H]Naltrindole from DOR in guinea pig brain membranes after 3 hrs by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 8.30nMAssay Description:Displacement of [3H]Naltrindole from DOR in guinea pig brain membranes after 3 hrs by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 9.80nMAssay Description:Displacement of [3H]Naltrindole from DOR in guinea pig brain membranes after 3 hrs by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 9.80nMAssay Description:Displacement of [3H]Naltrindole from DOR in guinea pig brain membranesMore data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Displacement of [3H]naltrindole from DOR in monkey brain cortex membranesMore data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:Displacement of [3H]Naltrindole from DOR in guinea pig brain membranes after 3 hrs by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 21nMAssay Description:Displacement of [3H]Naltrindole from DOR in guinea pig brain membranes after 3 hrs by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 21nMAssay Description:Displacement of [3H]naltrindole from DOR in monkey brain cortex membranesMore data for this Ligand-Target Pair
Affinity DataKi: 37nMAssay Description:Displacement of [3H]Naltrindole from DOR in guinea pig brain membranesMore data for this Ligand-Target Pair
Affinity DataKi: 52nMAssay Description:Displacement of [3H]Naltrindole from DOR in guinea pig brain membranesMore data for this Ligand-Target Pair
TargetOpioid receptor homologue(Danio rerio)
Instituto De Qu£Mica Avanzada De Catalu£A (Iqac-Csic)
Curated by ChEMBL
Instituto De Qu£Mica Avanzada De Catalu£A (Iqac-Csic)
Curated by ChEMBL
Affinity DataKi: 73nMAssay Description:Displacement of [3H]DPN from zebrafish delta 1a opioid receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
TargetOpioid receptor homologue(Danio rerio)
Instituto De Qu£Mica Avanzada De Catalu£A (Iqac-Csic)
Curated by ChEMBL
Instituto De Qu£Mica Avanzada De Catalu£A (Iqac-Csic)
Curated by ChEMBL
Affinity DataKi: 223nMAssay Description:Displacement of [3H]DPN from zebrafish delta 1a opioid receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
TargetOpioid receptor homologue(Danio rerio)
Instituto De Qu£Mica Avanzada De Catalu£A (Iqac-Csic)
Curated by ChEMBL
Instituto De Qu£Mica Avanzada De Catalu£A (Iqac-Csic)
Curated by ChEMBL
Affinity DataKi: 390nMAssay Description:Displacement of [3H]DPN from zebrafish delta 1a opioid receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
TargetOpioid receptor homologue(Danio rerio)
Instituto De Qu£Mica Avanzada De Catalu£A (Iqac-Csic)
Curated by ChEMBL
Instituto De Qu£Mica Avanzada De Catalu£A (Iqac-Csic)
Curated by ChEMBL
Affinity DataKi: 468nMAssay Description:Displacement of [3H]DPN from zebrafish delta 1a opioid receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: >500nMAssay Description:Displacement of [3H]DPDPE from delta opioid receptor in calf frontal cortex after 1 hr by gamma counterMore data for this Ligand-Target Pair
TargetOpioid receptor homologue(Danio rerio)
Instituto De Qu£Mica Avanzada De Catalu£A (Iqac-Csic)
Curated by ChEMBL
Instituto De Qu£Mica Avanzada De Catalu£A (Iqac-Csic)
Curated by ChEMBL
Affinity DataKi: 566nMAssay Description:Displacement of [3H]DPN from zebrafish delta 1a opioid receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
TargetOpioid receptor homologue(Danio rerio)
Instituto De Qu£Mica Avanzada De Catalu£A (Iqac-Csic)
Curated by ChEMBL
Instituto De Qu£Mica Avanzada De Catalu£A (Iqac-Csic)
Curated by ChEMBL
Affinity DataKi: 609nMAssay Description:Displacement of [3H]DPN from zebrafish delta 1a opioid receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
TargetOpioid receptor homologue(Danio rerio)
Instituto De Qu£Mica Avanzada De Catalu£A (Iqac-Csic)
Curated by ChEMBL
Instituto De Qu£Mica Avanzada De Catalu£A (Iqac-Csic)
Curated by ChEMBL
Affinity DataKi: 635nMAssay Description:Displacement of [3H]DPN from zebrafish delta 1a opioid receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
TargetOpioid receptor homologue(Danio rerio)
Instituto De Qu£Mica Avanzada De Catalu£A (Iqac-Csic)
Curated by ChEMBL
Instituto De Qu£Mica Avanzada De Catalu£A (Iqac-Csic)
Curated by ChEMBL
Affinity DataKi: 843nMAssay Description:Displacement of [3H]DPN from zebrafish delta 1a opioid receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.60E+3nMAssay Description:Displacement of [3H]DPDPE from delta-opioid receptor in guinea pig brain membranes after 30 mins by scintillation counting methodMore data for this Ligand-Target Pair