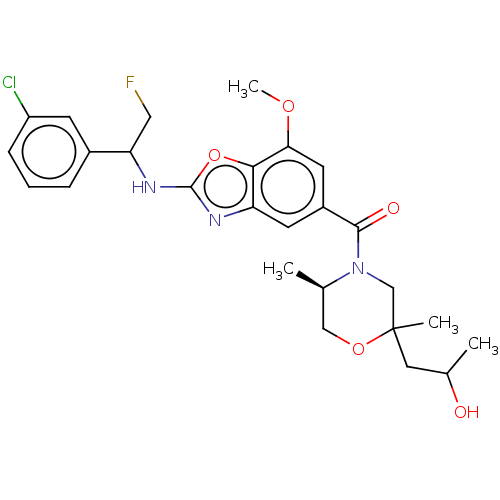
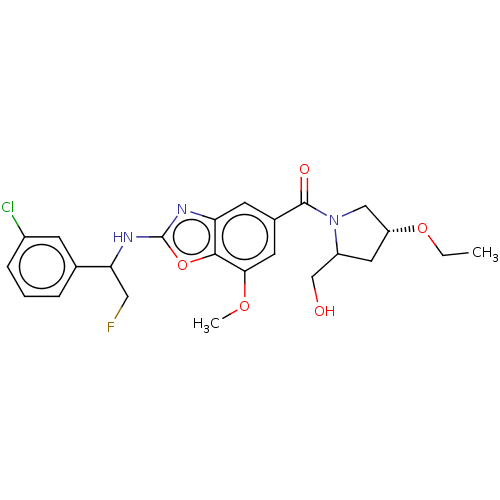
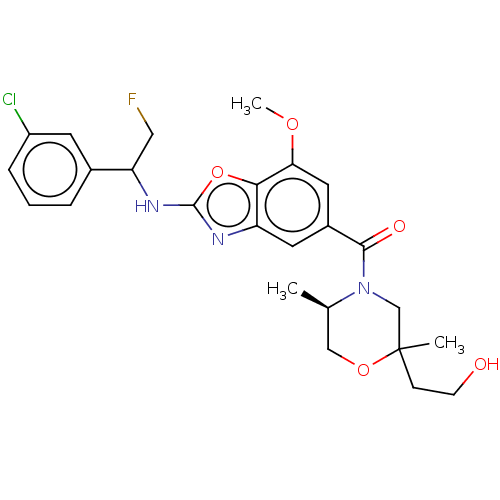
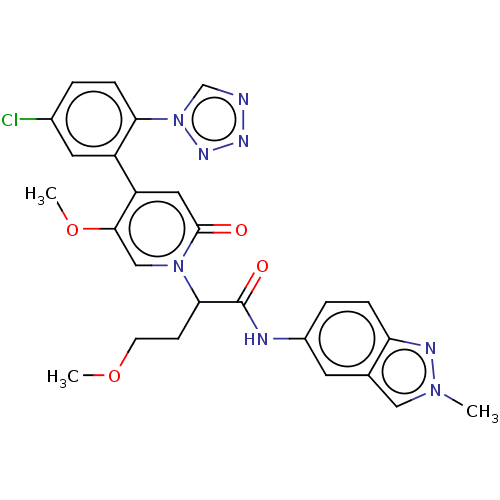
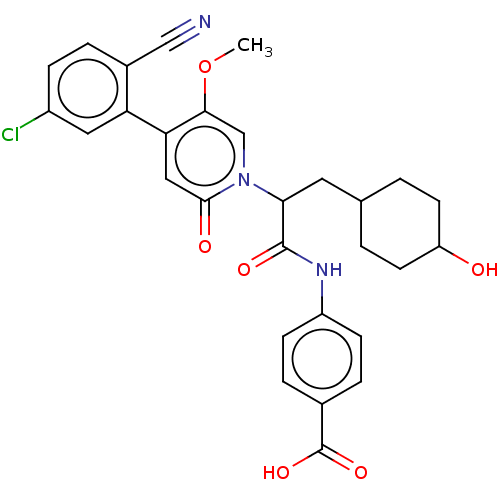
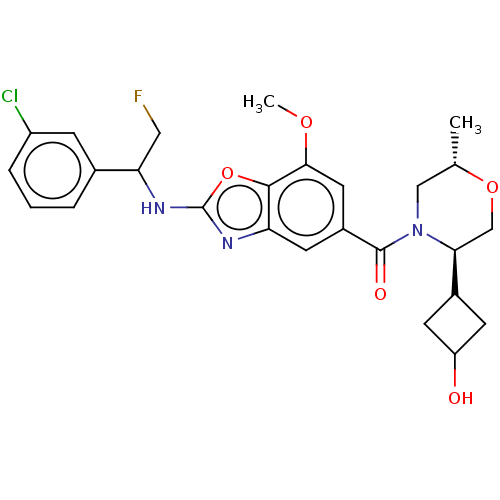
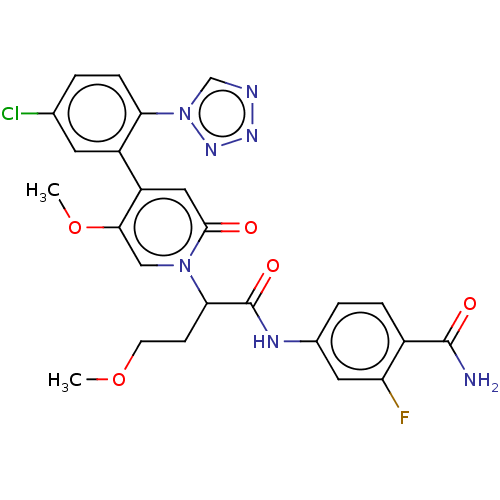
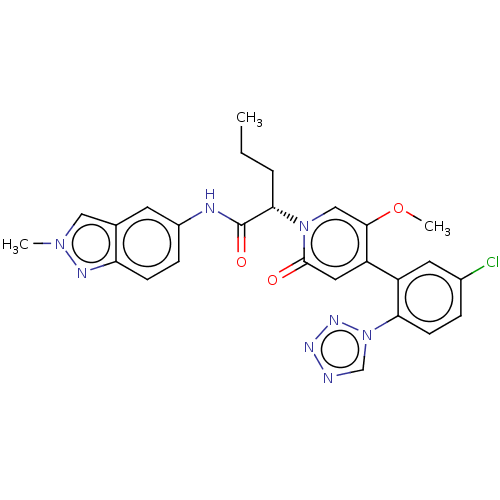
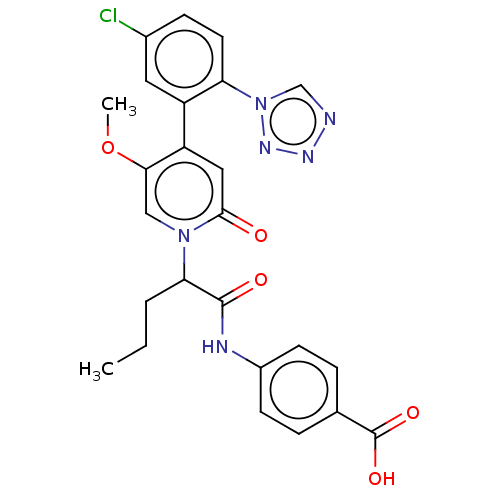
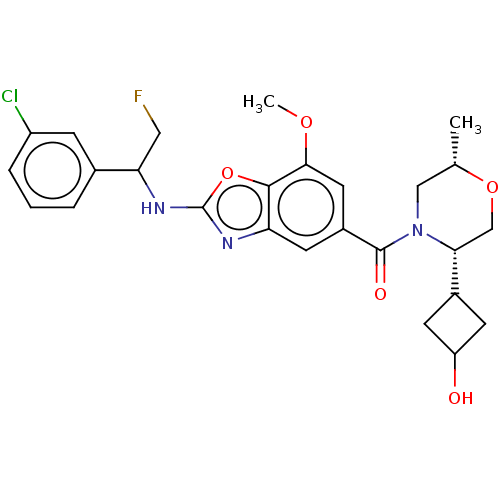
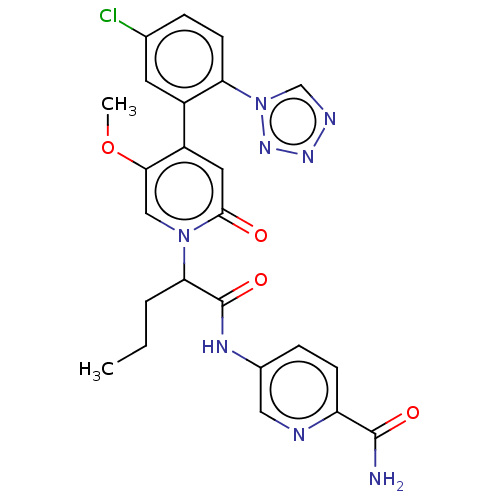
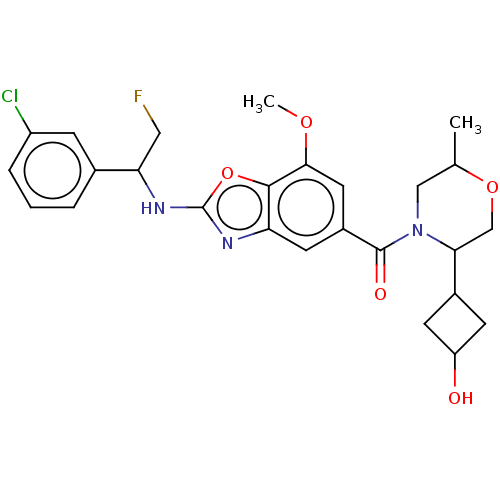
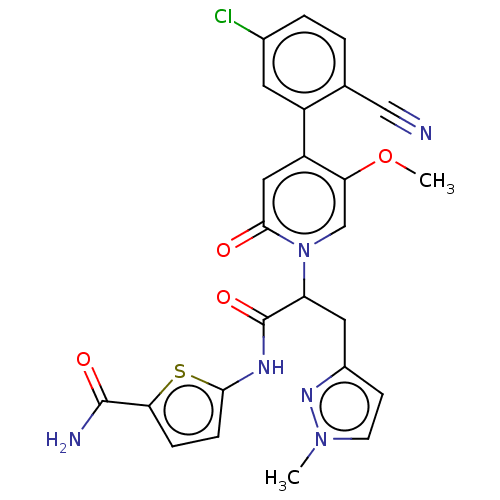
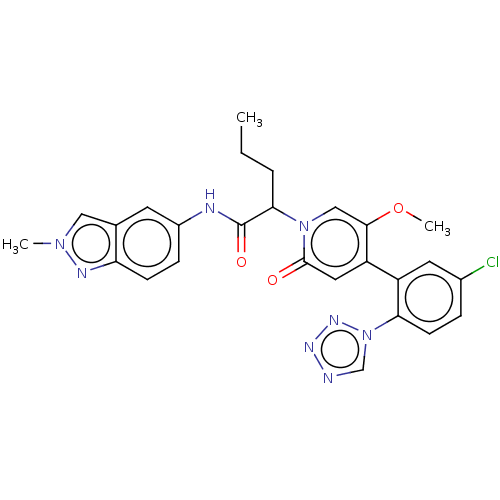
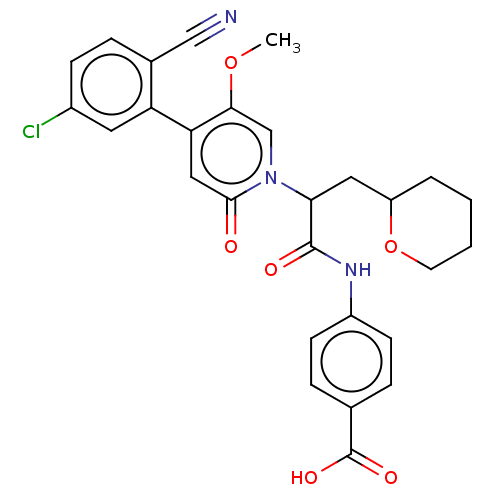
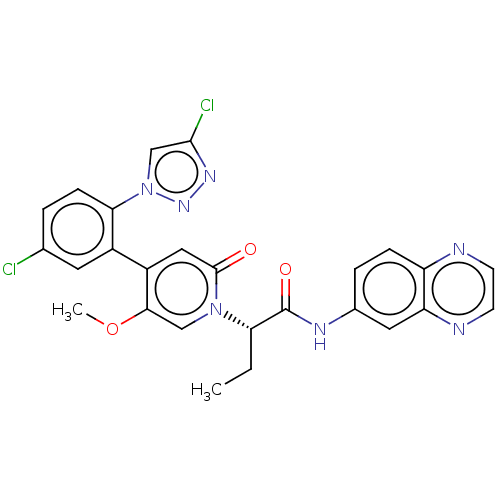
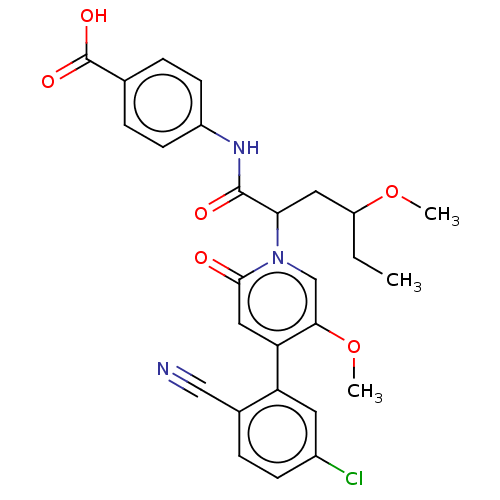
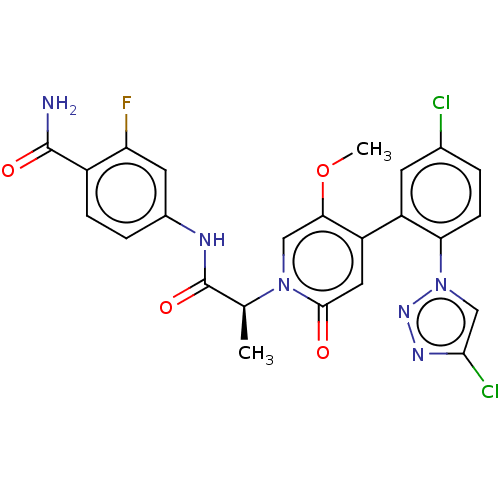
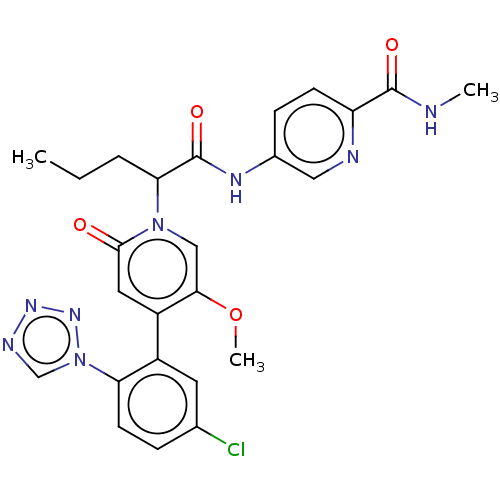
Affinity DataIC50: 0.0100nMpH: 7.4 T: 2°CAssay Description:To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMpH: 7.4 T: 2°CAssay Description:To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s...More data for this Ligand-Target Pair
Affinity DataIC50: 0.160nMpH: 7.4 T: 2°CAssay Description:To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s...More data for this Ligand-Target Pair
Affinity DataIC50: 0.190nMpH: 7.4 T: 2°CAssay Description:To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMpH: 7.4 T: 2°CAssay Description:To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s...More data for this Ligand-Target Pair
Affinity DataIC50: 0.260nMAssay Description:The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.260nMAssay Description:The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMpH: 7.4 T: 2°CAssay Description:Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con...More data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con...More data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:To determine the factor XIa inhibition of the substances according to the invention, a biochemical test system is used which utilizes the reaction of...More data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMpH: 7.4 T: 2°CAssay Description:To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s...More data for this Ligand-Target Pair
Affinity DataIC50: 0.310nMAssay Description:The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.340nMAssay Description:The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.340nMAssay Description:The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.360nMAssay Description:The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.360nMAssay Description:The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.360nMAssay Description:The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.370nMpH: 7.4 T: 2°CAssay Description:To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s...More data for this Ligand-Target Pair
Affinity DataIC50: 0.380nMpH: 7.4 T: 2°CAssay Description:To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.460nMAssay Description:The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.470nMpH: 7.4 T: 2°CAssay Description:To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s...More data for this Ligand-Target Pair
Affinity DataIC50: 0.490nMpH: 7.4 T: 2°CAssay Description:Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMpH: 7.4 T: 2°CAssay Description:Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:To determine the factor XIa inhibition of the substances according to the invention, a biochemical test system is used which utilizes the reaction of...More data for this Ligand-Target Pair
Affinity DataIC50: 0.530nMAssay Description:The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.540nMpH: 7.4 T: 2°CAssay Description:To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s...More data for this Ligand-Target Pair
Affinity DataIC50: 0.550nMAssay Description:The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.550nMAssay Description:The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.560nMAssay Description:The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.570nMAssay Description:To determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the reac...More data for this Ligand-Target Pair
Affinity DataIC50: 0.590nMAssay Description:The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMpH: 7.4 T: 2°CAssay Description:Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:To determine the factor XIa inhibition of the substances according to the invention, a biochemical test system is used which utilizes the reaction of...More data for this Ligand-Target Pair
Affinity DataIC50: 0.620nMAssay Description:The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.640nMAssay Description:The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.650nMAssay Description:The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.650nMAssay Description:The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.650nMAssay Description:The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.660nMAssay Description:The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMT: 2°CAssay Description:The enzymatic activity of human factor Xa (FXa) was measured using the conversion of a chromogenic substrate specific for FXa. Factor Xa cleaves p-ni...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMpH: 7.4 T: 2°CAssay Description:Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:To determine the factor XIa inhibition of the substances according to the invention, a biochemical test system is used which utilizes the reaction of...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con...More data for this Ligand-Target Pair