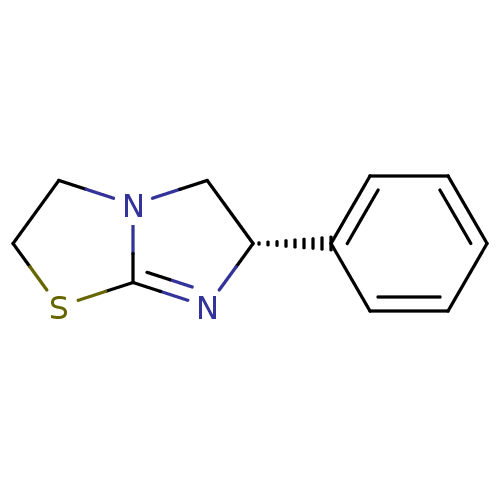
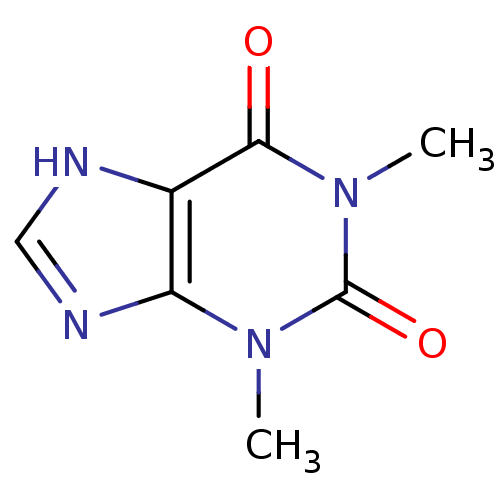
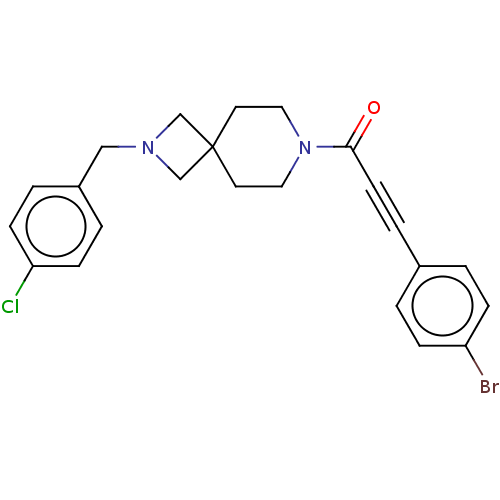
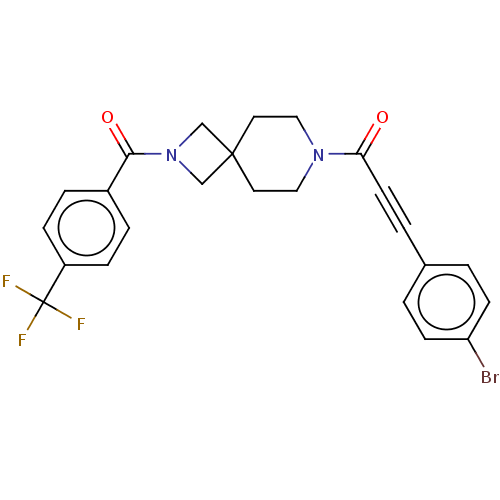
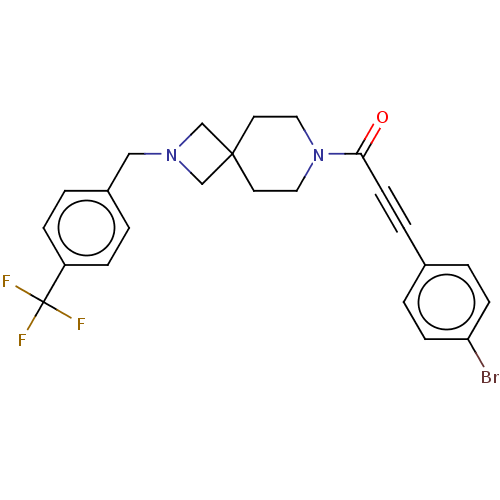
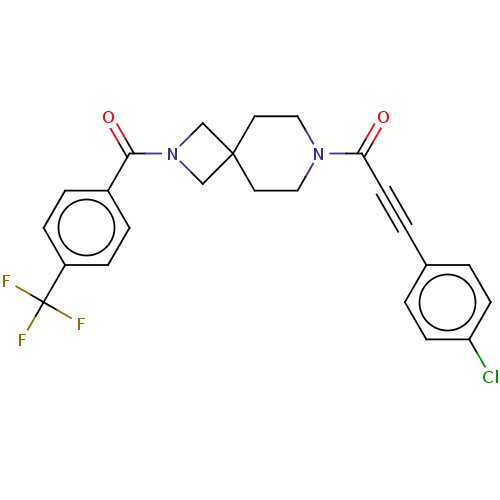
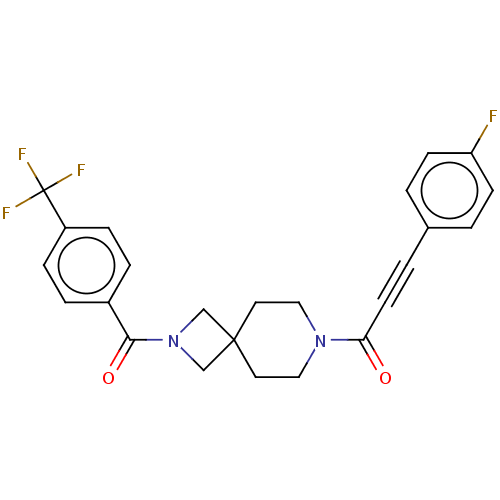
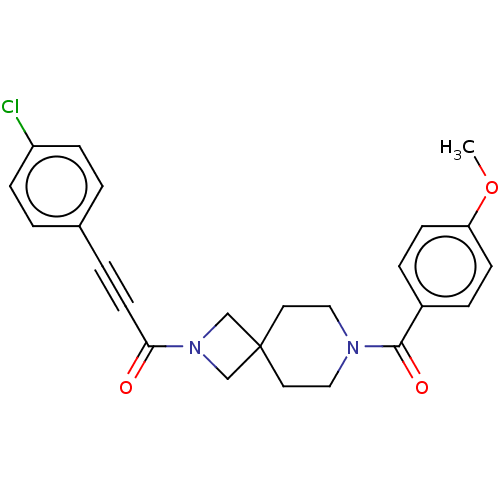
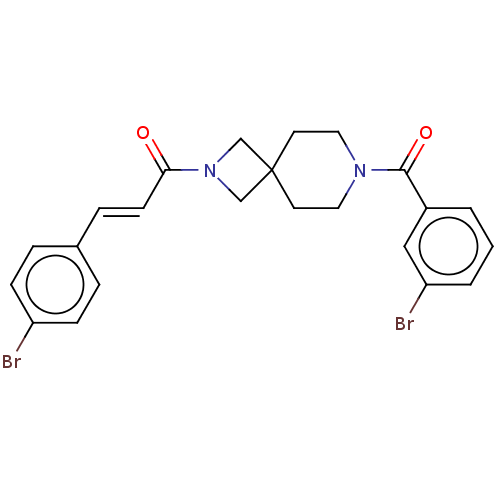
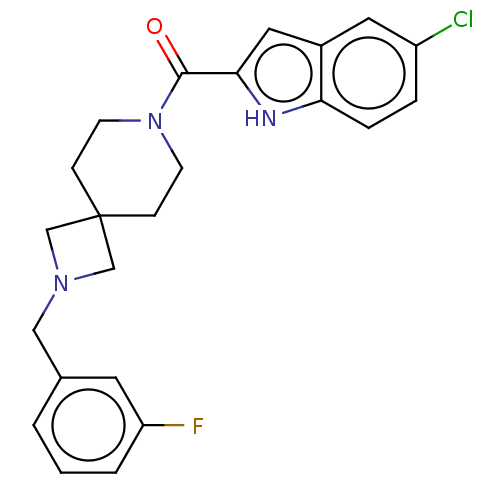
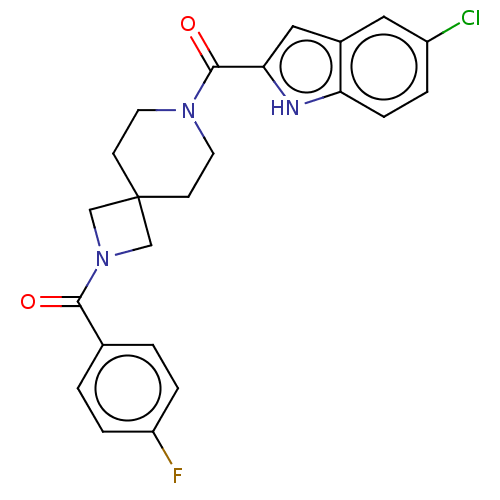
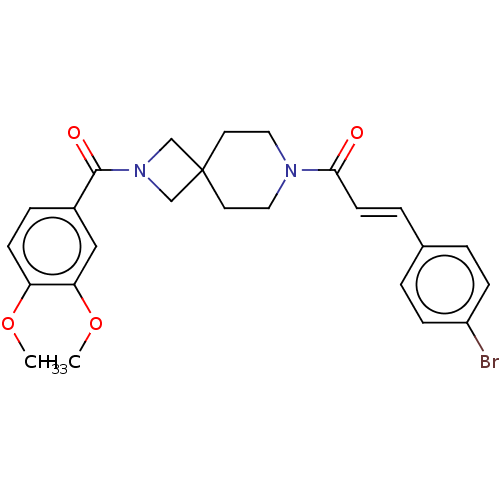
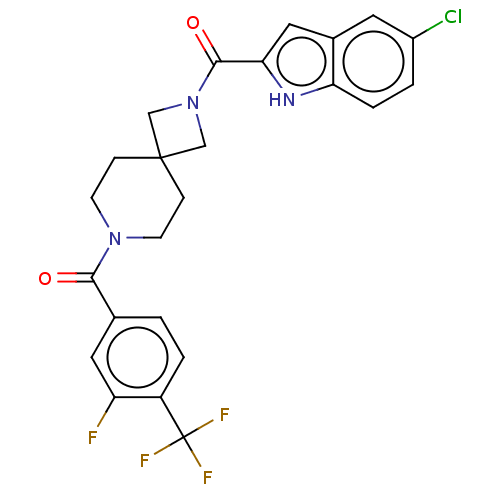
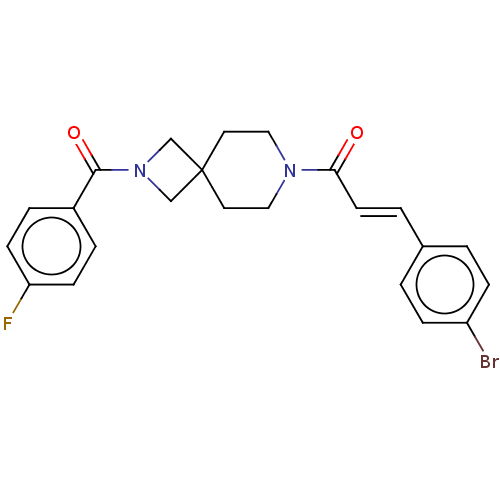
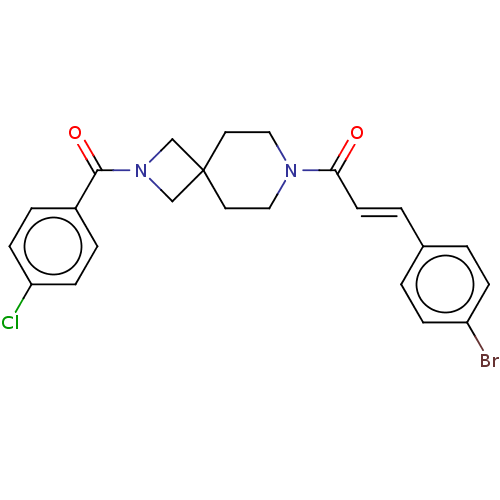
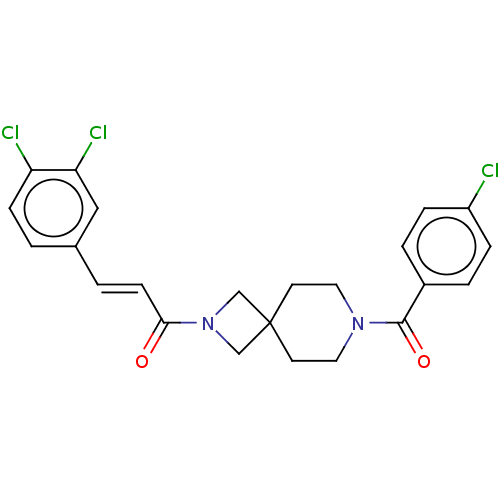
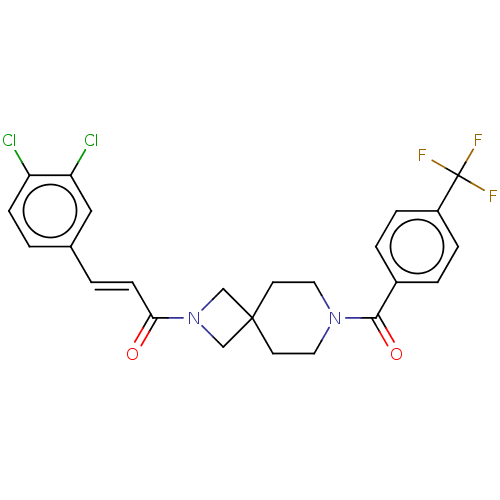
TargetAlkaline phosphatase, tissue-nonspecific isozyme(Homo sapiens (Human))
Institute For Medical Research
Curated by ChEMBL
Institute For Medical Research
Curated by ChEMBL
Affinity DataKi: 340nMAssay Description:Inhibition of TNAP using CDP-star as substrate by non-competitive Lineweaver-Burk plotMore data for this Ligand-Target Pair
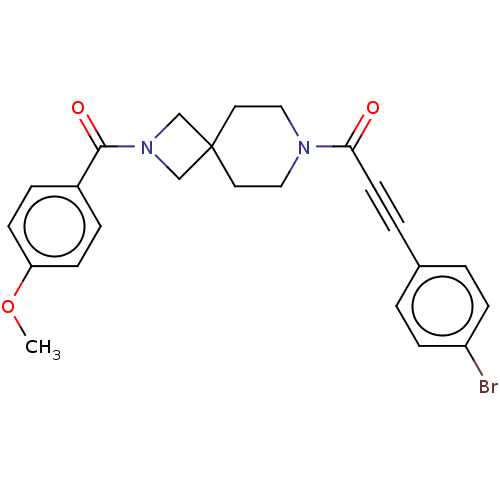
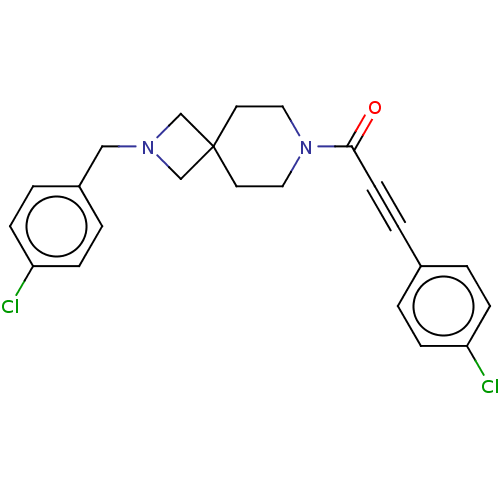
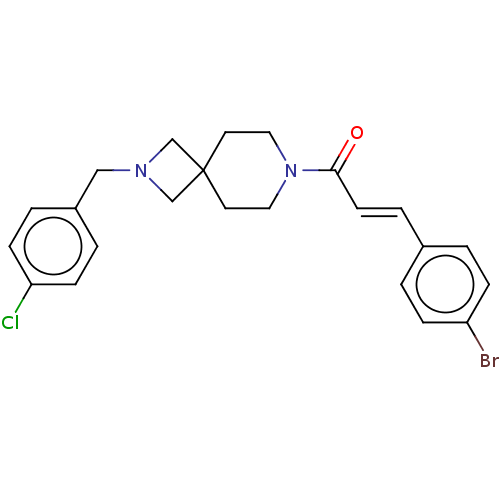
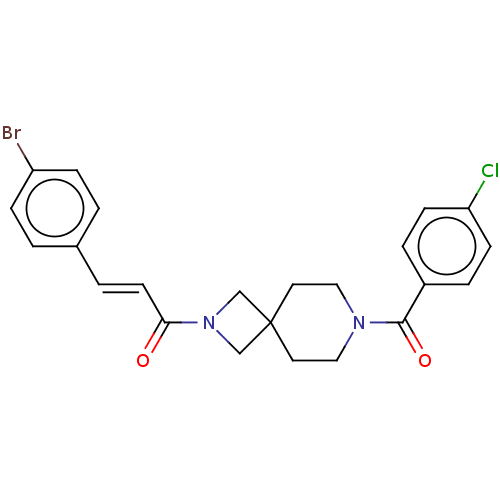
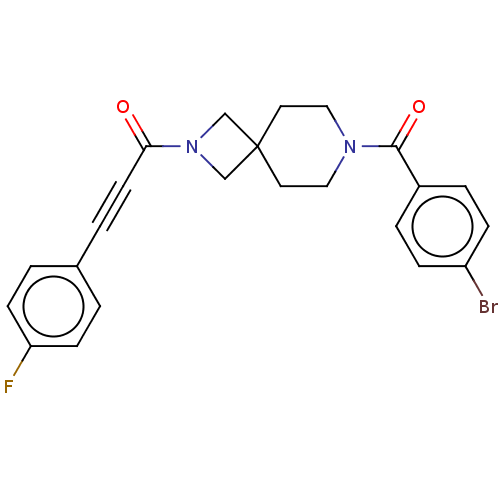
TargetAlkaline phosphatase, tissue-nonspecific isozyme(Homo sapiens (Human))
Institute For Medical Research
Curated by ChEMBL
Institute For Medical Research
Curated by ChEMBL
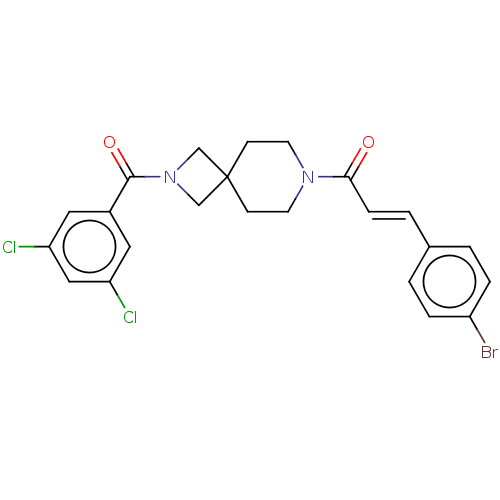
Affinity DataKi: 590nMAssay Description:Inhibition of TNAP using DEA as substrate by non-competitive Lineweaver-Burk plotMore data for this Ligand-Target Pair
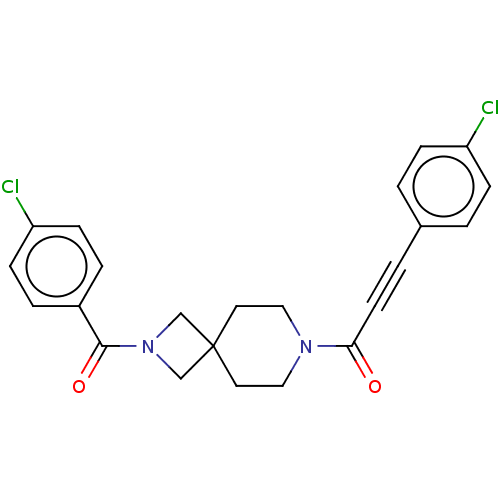
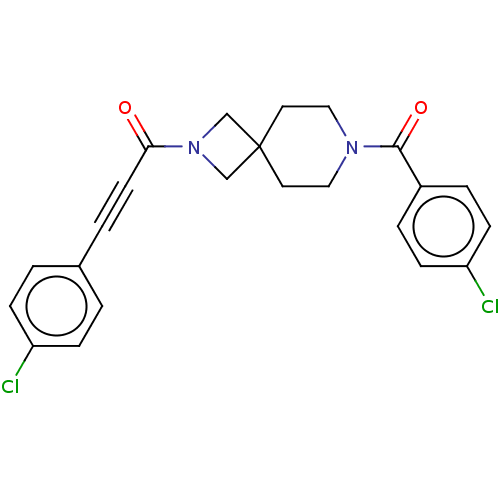
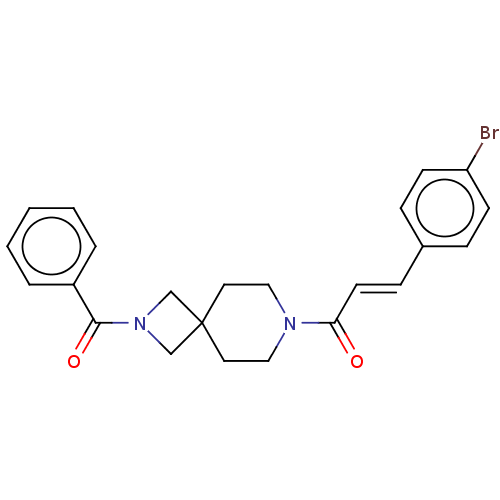
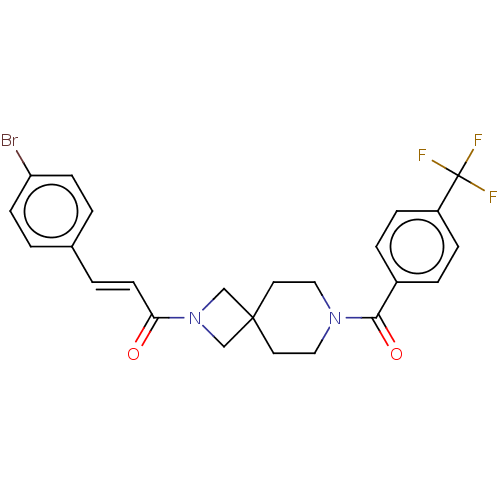
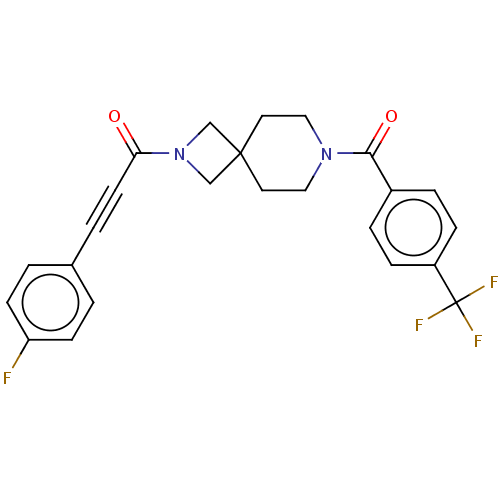
TargetTyrosine-protein phosphatase non-receptor type 7(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
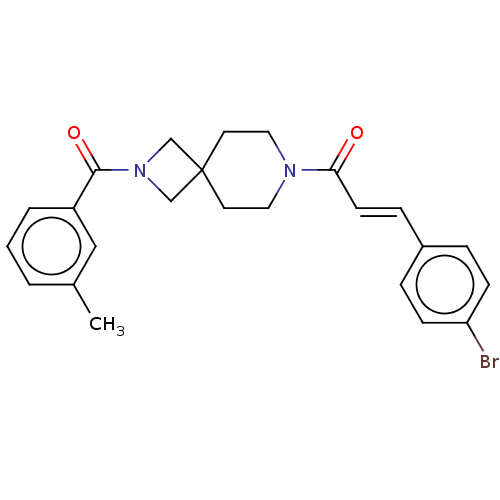
Affinity DataKi: 690nMAssay Description:Competitive inhibition of recombinant HePTP expressed in Escherichia coli by Michaelis-Menten kinetic analysisMore data for this Ligand-Target Pair
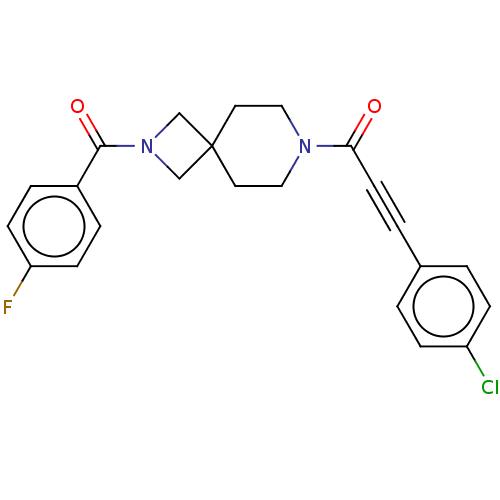
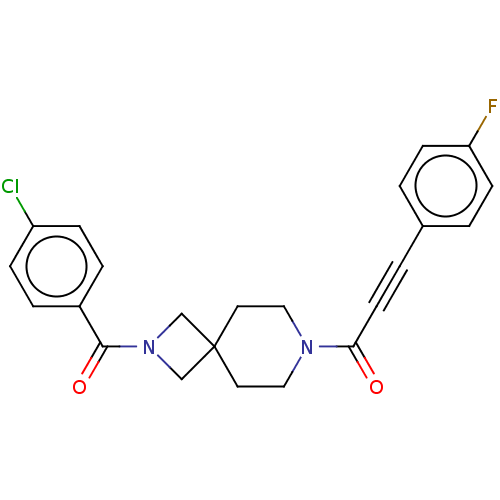
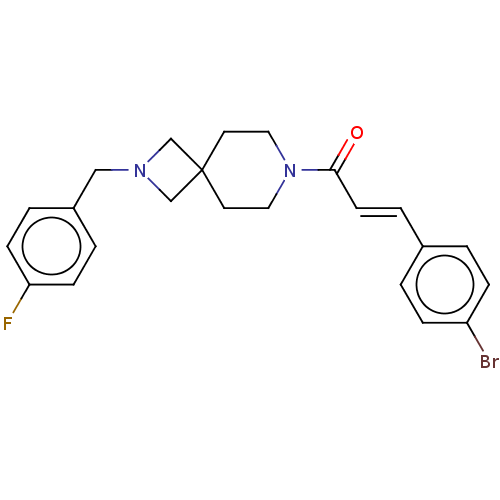
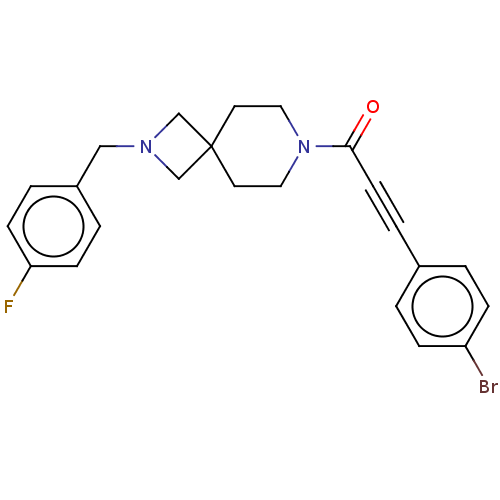
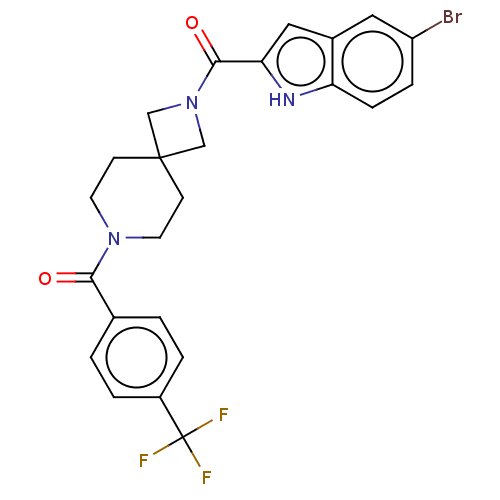
TargetIntestinal-type alkaline phosphatase(Mus musculus)
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
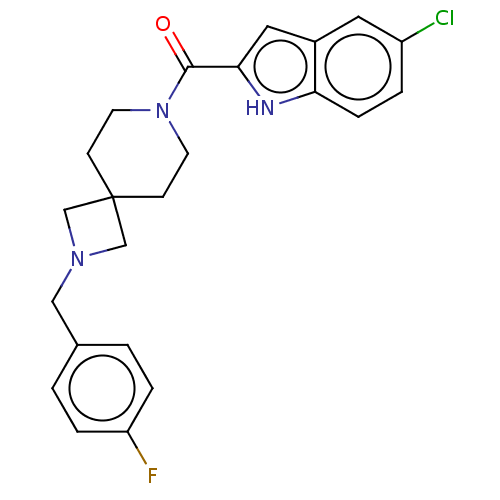
Affinity DataKi: 3.20E+3nMAssay Description:Competitive inhibition of mouse duodenal-specific FLAG-tagged IAP expressed in African green monkey COS1 cells using p-nitrophenyl phosphate as subst...More data for this Ligand-Target Pair
TargetAlkaline phosphatase, tissue-nonspecific isozyme(Homo sapiens (Human))
Institute For Medical Research
Curated by ChEMBL
Institute For Medical Research
Curated by ChEMBL
Affinity DataKi: 1.60E+4nMAssay Description:Inhibition of TNSALP (unknown origin)More data for this Ligand-Target Pair
TargetAlkaline phosphatase, tissue-nonspecific isozyme(Homo sapiens (Human))
Institute For Medical Research
Curated by ChEMBL
Institute For Medical Research
Curated by ChEMBL
Affinity DataKi: 8.20E+4nMAssay Description:Inhibition of TNSALP (unknown origin)More data for this Ligand-Target Pair
TargetAlkaline phosphatase, tissue-nonspecific isozyme(Homo sapiens (Human))
Institute For Medical Research
Curated by ChEMBL
Institute For Medical Research
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of TNSALP (unknown origin)More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair