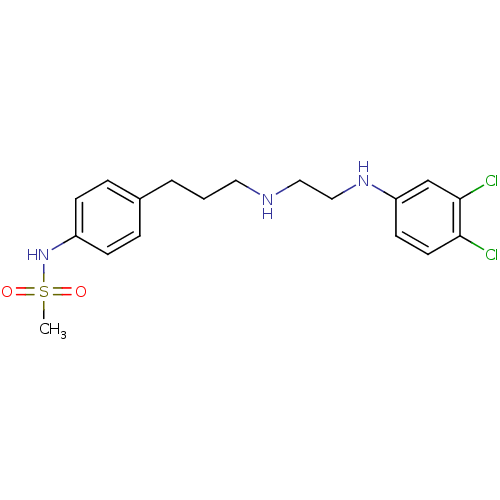
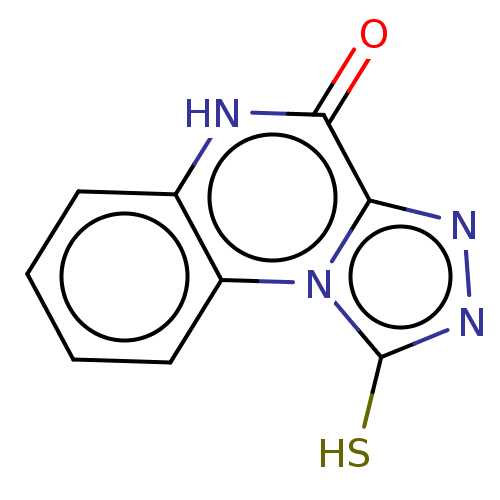
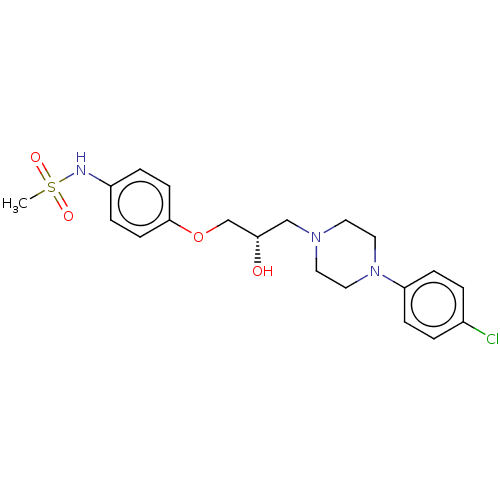
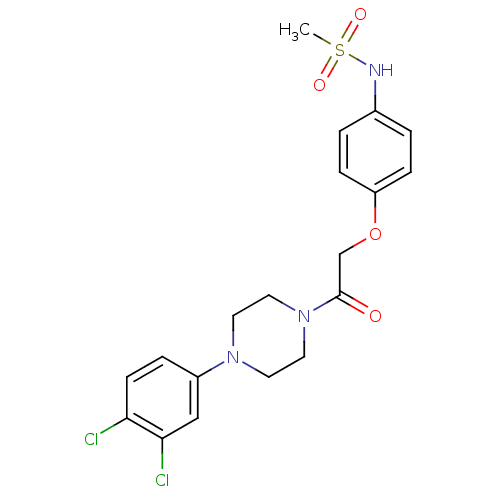
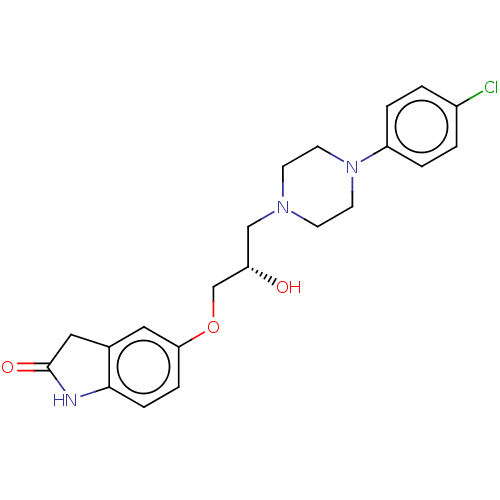
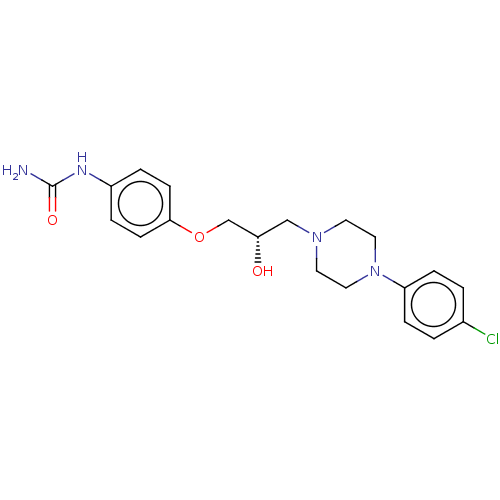
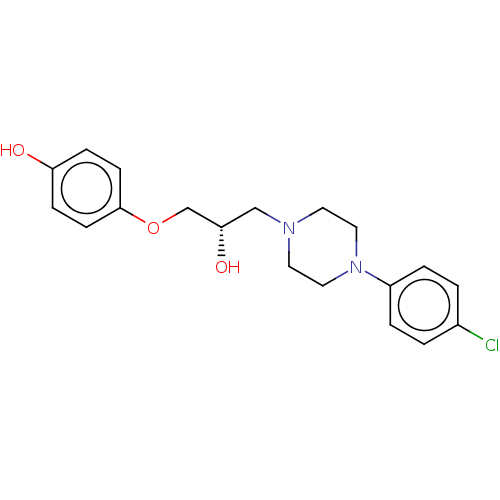
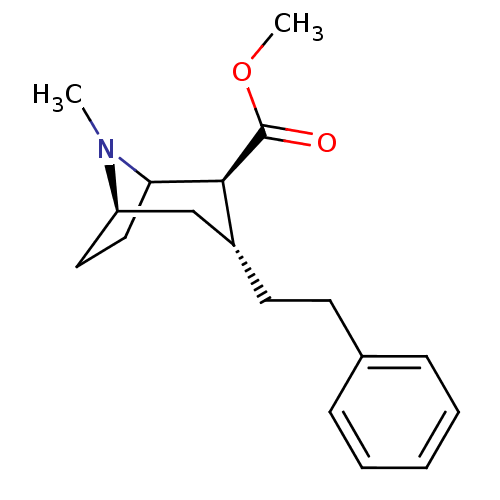
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Emory University
Curated by ChEMBL
Emory University
Curated by ChEMBL
Affinity DataKi: 14nMAssay Description:Displacement of [3H]astemizole from human recombinant ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 65nM ΔG°: -41.0kJ/mole IC50: 92nMpH: 7.4 T: 2°CAssay Description:Two-electrode voltage clamp (TEVC) recordings were performed on Xenopus oocytes at room temperature 3-6 days postinjection using an OC-725C TEVC ampl...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Rattus norvegicus (Rat))
Emory University
Curated by ChEMBL
Emory University
Curated by ChEMBL
Affinity DataKi: 119nMAssay Description:Displacement of [3H]ifenprodil form NR2B receptor in Wistar rat cerebral cortex membraneMore data for this Ligand-Target Pair
Affinity DataKi: >300nM ΔG°: >-37.2kJ/mole IC50: >3.00E+3nMpH: 7.4 T: 2°CAssay Description:Two-electrode voltage clamp (TEVC) recordings were performed on Xenopus oocytes at room temperature 3-6 days postinjection using an OC-725C TEVC ampl...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Emory University
Curated by ChEMBL
Emory University
Curated by ChEMBL
Affinity DataKi: 300nMAssay Description:Displacement of [3H]astemizole from human recombinant ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Rattus norvegicus (Rat))
Emory University
Curated by ChEMBL
Emory University
Curated by ChEMBL
Affinity DataKi: 300nMAssay Description:Displacement of [3H]ifenprodil form NR2B receptor in Wistar rat cerebral cortex membraneMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Emory University
Curated by ChEMBL
Emory University
Curated by ChEMBL
Affinity DataKi: 553nMAssay Description:Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole...More data for this Ligand-Target Pair
Affinity DataKi: 560nM ΔG°: -35.7kJ/mole IC50: 770nMpH: 7.4 T: 2°CAssay Description:Two-electrode voltage clamp (TEVC) recordings were performed on Xenopus oocytes at room temperature 3-6 days postinjection using an OC-725C TEVC ampl...More data for this Ligand-Target Pair
Affinity DataKi: >800nM ΔG°: >-34.8kJ/mole IC50: >3.00E+3nMpH: 7.4 T: 2°CAssay Description:Two-electrode voltage clamp (TEVC) recordings were performed on Xenopus oocytes at room temperature 3-6 days postinjection using an OC-725C TEVC ampl...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Rattus norvegicus (Rat))
Emory University
Curated by ChEMBL
Emory University
Curated by ChEMBL
Affinity DataKi: 817nMAssay Description:Displacement of [3H]ifenprodil form NR2B receptor in Wistar rat cerebral cortex membraneMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Emory University
Curated by ChEMBL
Emory University
Curated by ChEMBL
Affinity DataKi: 1.00E+3nMAssay Description:Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole...More data for this Ligand-Target Pair
Affinity DataKi: >1.30E+3nM ΔG°: >-33.6kJ/mole IC50: >3.00E+3nMpH: 7.4 T: 2°CAssay Description:Two-electrode voltage clamp (TEVC) recordings were performed on Xenopus oocytes at room temperature 3-6 days postinjection using an OC-725C TEVC ampl...More data for this Ligand-Target Pair
TargetC-terminal processing protease of the D1 protein(Spinacia oleracea)
Central China Normal University
Curated by ChEMBL
Central China Normal University
Curated by ChEMBL
Affinity DataKi: 1.30E+3nMAssay Description:Competitive inhibition of CtpA in Spinacia oleracea (spinach) thylakoids using S24 substrate incubated for 30 min by HPLC method based double-recipro...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Emory University
Curated by ChEMBL
Emory University
Curated by ChEMBL
Affinity DataKi: 1.60E+3nMAssay Description:Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Emory University
Curated by ChEMBL
Emory University
Curated by ChEMBL
Affinity DataKi: 3.00E+3nMAssay Description:Displacement of [3H]astemizole from human recombinant ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Emory University
Curated by ChEMBL
Emory University
Curated by ChEMBL
Affinity DataKi: 4.62E+3nMAssay Description:Displacement of [3H]astemizole from human recombinant ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Emory University
Curated by ChEMBL
Emory University
Curated by ChEMBL
Affinity DataKi: 5.00E+3nMAssay Description:Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Emory University
Curated by ChEMBL
Emory University
Curated by ChEMBL
Affinity DataKi: 7.50E+3nMAssay Description:Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Emory University
Curated by ChEMBL
Emory University
Curated by ChEMBL
Affinity DataKi: 7.50E+3nMAssay Description:Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Emory University
Curated by ChEMBL
Emory University
Curated by ChEMBL
Affinity DataKi: 7.50E+3nMAssay Description:Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Inhibition of histamine H2 receptor (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Emory University
Curated by ChEMBL
Emory University
Curated by ChEMBL
Affinity DataKi: 1.00E+4nMAssay Description:Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Emory University
Curated by ChEMBL
Emory University
Curated by ChEMBL
Affinity DataKi: 1.30E+4nMAssay Description:Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1 [F484A,T518L]/3A(Rattus norvegicus (Rat))
University of Copenhagen
University of Copenhagen
Affinity DataKi: 3.50E+4nM ΔG°: -25.4kJ/mole IC50: 9.70E+4nMpH: 7.4 T: 2°CAssay Description:Two-electrode voltage clamp (TEVC) recordings were performed on Xenopus oocytes at room temperature 3-6 days postinjection using an OC-725C TEVC ampl...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Emory University
Curated by ChEMBL
Emory University
Curated by ChEMBL
Affinity DataKi: 3.90E+4nMAssay Description:Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1 [F484A,T518L]/3B(Rattus norvegicus (Rat))
University of Copenhagen
University of Copenhagen
Affinity DataKi: >1.50E+5nM ΔG°: >-21.8kJ/mole IC50: >3.00E+5nMpH: 7.4 T: 2°CAssay Description:Two-electrode voltage clamp (TEVC) recordings were performed on Xenopus oocytes at room temperature 3-6 days postinjection using an OC-725C TEVC ampl...More data for this Ligand-Target Pair
Affinity DataKi: >1.80E+5nM ΔG°: >-21.4kJ/mole IC50: >3.00E+5nMpH: 7.4 T: 2°CAssay Description:Two-electrode voltage clamp (TEVC) recordings were performed on Xenopus oocytes at room temperature 3-6 days postinjection using an OC-725C TEVC ampl...More data for this Ligand-Target Pair
Target11-beta-hydroxysteroid dehydrogenase type 2(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of human 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [3H]cortisone into [3H]cortisol by scintillation proximity assayMore data for this Ligand-Target Pair
TargetSodium-dependent dopamine transporter(Rattus norvegicus (rat))
University Of Maryland
Curated by ChEMBL
University Of Maryland
Curated by ChEMBL
Affinity DataIC50: 2.10nMAssay Description:Inhibition [3H]-cocaine binding to Cocaine receptor in rat striatal membranes.More data for this Ligand-Target Pair
Target11-beta-hydroxysteroid dehydrogenase 1(Mus musculus (mouse))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of mouse 11beta-HSD1 expressed in HEK293 cells assessed as conversion of [3H]cortisone into [3H]cortisol by scintillation proximity assayMore data for this Ligand-Target Pair
TargetSodium-dependent dopamine transporter(Rattus norvegicus (rat))
University Of Maryland
Curated by ChEMBL
University Of Maryland
Curated by ChEMBL
Affinity DataIC50: 3.30nMAssay Description:Inhibition [3H]-cocaine binding to Cocaine receptor in rat striatal membranes.More data for this Ligand-Target Pair
TargetSodium-dependent dopamine transporter(Rattus norvegicus (rat))
University Of Maryland
Curated by ChEMBL
University Of Maryland
Curated by ChEMBL
Affinity DataIC50: 3.5nMAssay Description:Inhibition [3H]DA uptake in rat striatal synaptosomes.More data for this Ligand-Target Pair
TargetSodium-dependent dopamine transporter(Rattus norvegicus (rat))
University Of Maryland
Curated by ChEMBL
University Of Maryland
Curated by ChEMBL
Affinity DataIC50: 5.90nMAssay Description:Inhibition [3H]DA uptake in rat striatal synaptosomes.More data for this Ligand-Target Pair
Target11-beta-hydroxysteroid dehydrogenase 1(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of human 11beta-HSD1 expressed in HEK293 cells assessed as conversion of [3H]cortisone into [3H]cortisol by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Inhibition of recombinant aurora AMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of recombinant aurora AMore data for this Ligand-Target Pair
TargetSodium-dependent dopamine transporter(Rattus norvegicus (rat))
University Of Maryland
Curated by ChEMBL
University Of Maryland
Curated by ChEMBL
Affinity DataIC50: 9.90nMAssay Description:Inhibition [3H]-cocaine binding to Cocaine receptor in rat striatal membranes.More data for this Ligand-Target Pair
TargetSodium-dependent dopamine transporter(Rattus norvegicus (rat))
University Of Maryland
Curated by ChEMBL
University Of Maryland
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibition [3H]-cocaine binding to Cocaine receptor in rat striatal membranes.More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibition of recombinant aurora AMore data for this Ligand-Target Pair
Target11-beta-hydroxysteroid dehydrogenase 1(Mus musculus (mouse))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of mouse 11beta-HSD1 expressed in HEK293 cells assessed as conversion of [3H]cortisone into [3H]cortisol by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibition of recombinant aurora AMore data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Inhibition of recombinant aurora AMore data for this Ligand-Target Pair
Affinity DataIC50: 29nMAssay Description:Inhibition of recombinant aurora AMore data for this Ligand-Target Pair
TargetSodium-dependent dopamine transporter(Rattus norvegicus (rat))
University Of Maryland
Curated by ChEMBL
University Of Maryland
Curated by ChEMBL
Affinity DataIC50: 29nMAssay Description:Inhibition [3H]DA uptake in rat striatal synaptosomes.More data for this Ligand-Target Pair
Affinity DataIC50: 31nMAssay Description:Inhibition of recombinant aurora AMore data for this Ligand-Target Pair
Affinity DataIC50: 34nMAssay Description:Inhibition of recombinant aurora AMore data for this Ligand-Target Pair
Affinity DataIC50: 34nMpH: 7.4 T: 2°CAssay Description:Two-electrode voltage clamp (TEVC) recordings were performed on Xenopus oocytes at room temperature 3-6 days postinjection using an OC-725C TEVC ampl...More data for this Ligand-Target Pair
Affinity DataIC50: 38nMAssay Description:Inhibition of rat recombinant NR1/NR2B receptor expressed in Xenopus oocytes assessed as inhibition of glutamate and glycine-induced evoked current b...More data for this Ligand-Target Pair
Affinity DataIC50: 39nMAssay Description:Inhibition of recombinant aurora AMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMpH: 7.4 T: 2°CAssay Description:Two-electrode voltage clamp (TEVC) recordings were performed on Xenopus oocytes at room temperature 3-6 days postinjection using an OC-725C TEVC ampl...More data for this Ligand-Target Pair