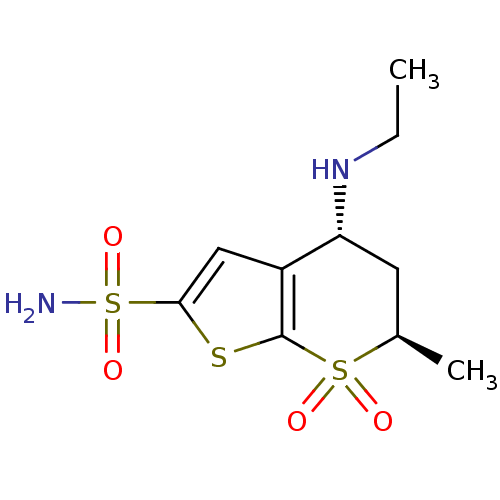
BDBM13054 (2R,4R)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H-1,7-thieno[2,3-b]thiopyran-6-sulfonamide::Dorzolamide, DZA::sulfonamide 5
SMILES CCN[C@@H]1C[C@@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O
InChI Key InChIKey=IAVUPMFITXYVAF-UHFFFAOYSA-N
Data 13 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 13 hits for monomerid = 13054
Found 13 hits for monomerid = 13054
Affinity DataKi: 3.5nMAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair
Affinity DataKi: 3.5nMAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair
Affinity DataKi: 9nM ΔG°: -10.9kcal/molepH: 7.5 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair
Affinity DataKi: 9nM ΔG°: -10.8kcal/molepH: 7.5 T: 2°CAssay Description:Inhibition assay using carbonic anhydrases with an SX.18MV-R, a stopped-flow instrument from Applied Photophysics was used for assaying the CA cataly...More data for this Ligand-Target Pair
Affinity DataKi: 10nM ΔG°: -10.7kcal/molepH: 7.5 T: 2°CAssay Description:Inhibition assay using carbonic anhydrases with an SX.18MV-R, a stopped-flow instrument from Applied Photophysics was used for assaying the CA cataly...More data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair
Affinity DataKi: 27nMAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair
Affinity DataKi: 33nMAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair
Affinity DataKi: 42nMAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair
Affinity DataKi: 52nMAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair
Affinity DataKi: 8.50E+3nMAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair
Affinity DataKi: 5.00E+4nM ΔG°: -5.77kcal/molepH: 7.5 T: 2°CAssay Description:Inhibition assay using carbonic anhydrases with an SX.18MV-R, a stopped-flow instrument from Applied Photophysics was used for assaying the CA cataly...More data for this Ligand-Target Pair
Affinity DataKi: 5.00E+4nM ΔG°: -5.80kcal/molepH: 7.5 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair