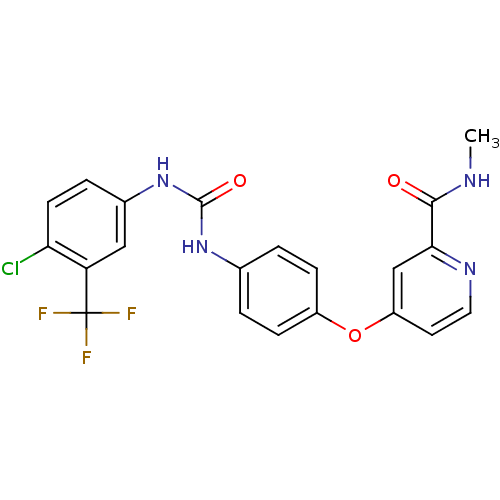
BDBM16673 4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamoyl}amino)phenoxy]-N-methylpyridine-2-carboxamide::4-[4-[[4-chloro-3-(trifluoromethyl)phenyl]carbamoylamino]phenoxy]-N-methyl-picolinamide;tosylic acid::BAY 43-9006::BAY 439006::BAY439006::CHEMBL1336::Hit compound, 8::Nexavar::Sorafenib::Sorafenib, 4::US10183928, Sorafenib::US10202365, Compound Sorafenib::US10227329, Compound Sorafenib::US10584114, Compound Sorafenib::US10774070, Compound Sorafenib::US10980809, Example Sorafenib::US11279688, Compound Sorafenib::US11505527, Compound Sorafenib::US11912663, Compound Sorafenib::US9029401, Sorafenib::US9469639, Sorafenib::US9902709, Comparative example 1::Xarelto::cid_216239
SMILES CNC(=O)c1cc(ccn1)Oc2ccc(cc2)NC(=O)Nc3ccc(c(c3)C(F)(F)F)Cl
InChI Key InChIKey=MLDQJTXFUGDVEO-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 1705 hits for monomerid = 16673
Found 1705 hits for monomerid = 16673
The First Affiliated Hospital of Xi'An Jiaotong University
Curated by ChEMBL
The First Affiliated Hospital of Xi'An Jiaotong University
Curated by ChEMBL
The First Affiliated Hospital of Xi'An Jiaotong University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Ambit Biosciences
Curated by PubChem BioAssay
Ambit Biosciences
Curated by PubChem BioAssay
Ambit Biosciences
Curated by PubChem BioAssay
Ambit Biosciences
Curated by PubChem BioAssay
Ambit Biosciences
Curated by PubChem BioAssay
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
National Taiwan University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
 3D Structure (crystal)
3D Structure (crystal)