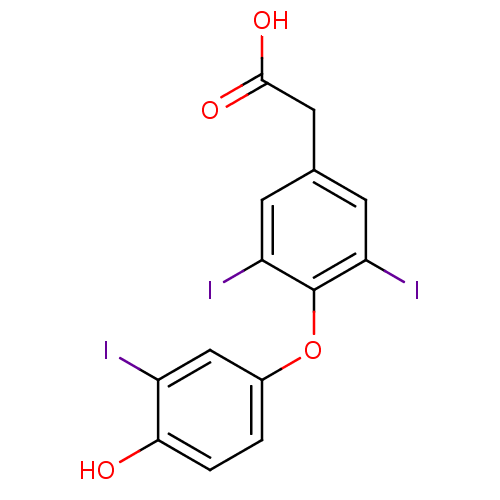
BDBM18862 2-[4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl]acetic acid::CHEMBL41632::TRIAC::Tiratricol::Triiodothyroacetic acid, 3::US10322118, Entry Tiratricol
SMILES c1cc(c(cc1Oc2c(cc(cc2I)CC(=O)O)I)I)O
InChI Key InChIKey=UOWZUVNAGUAEQC-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 18 hits for monomerid = 18862
Found 18 hits for monomerid = 18862
Affinity DataIC50: 0.0407nMAssay Description:Inhibition of thyroid hormone receptor betaMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0479nMAssay Description:Inhibition of human thyroid hormone receptor beta 1More data for this Ligand-Target Pair
Affinity DataIC50: 0.0480nMpH: 7.0 T: 2°CAssay Description:IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta.More data for this Ligand-Target Pair
Affinity DataIC50: 0.140nMpH: 7.0 T: 2°CAssay Description:IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. More data for this Ligand-Target Pair
Affinity DataIC50: 0.141nMAssay Description:Inhibition of thyroid hormone receptor alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 0.150nMAssay Description:In vitro inhibition of the bound [125I]L-T3 rat liver nuclear L-triiodothyronine receptorMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Human)
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataKd: 200nMAssay Description:Binding affinity to recombinant PPARgamma LBD (unknown origin) by isothermal titration calorimetryMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Human)
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataEC50: 370nMAssay Description:Agonist activity at recombinant human pFA-CMV fused PPARgamma expressed in HEK293T cells transfected with pFR-luciferase plasmid and pRL-SV40 plasmid...More data for this Ligand-Target Pair
Affinity DataKd: 1.32E+3nMAssay Description:Binding affinity to RXRalpha LBD (unknown origin) by isothermal titration calorimetryMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1C1(Mouse)
The University of Tokyo
Curated by ChEMBL
The University of Tokyo
Curated by ChEMBL
Affinity DataKi: 2.15E+3nMAssay Description:TP_TRANSPORTER: inhibition of L-T4 uptake in Oatp14-expressing HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 4.70E+3nMAssay Description:The first step in characterizing the two compounds identified by high throughput screening was to verify that the inhibition was reproducible using f...More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:In vitro inhibition of bound [125I]L-T3 rat plasma membrane 3,5,3'' L-triiodothyronine receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 6.90E+3nMAssay Description:Inhibition of HA-tagged human NTCP expressed in human U2OS cells assessed as reduction in [14C]taurocholate uptake preincubated for 10 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: 6.90E+3nMAssay Description:Inhibition of human NTCP mediated TCA uptake in U2OS expresseing HA-tagged NTCP cells preincubated for 10 mins followed by substrate addition and mea...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of preS1-peptide binding to human HA-tagged NTCP in U2OS expresseing NTCP in incubated for 24 hrs using Myrcludex B as substrate by compet...More data for this Ligand-Target Pair
Affinity DataEC50: 1.20E+4nMAssay Description:Agonist activity at recombinant human pFA-CMV fused RXRalpha LBD expressed in HEK293T cells transfected with pFR-luciferase plasmid and pRL-SV40 plas...More data for this Ligand-Target Pair
TargetProliferating cell nuclear antigen(Human)
St. Jude Children'S Research Hospital
Curated by ChEMBL
St. Jude Children'S Research Hospital
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of human recombinant PCNA interaction with PIP box protein N-5-carboxyfluorescein-SAVLQKKITDYFHPKK after 30 mins by fluorescence polarizat...More data for this Ligand-Target Pair
Affinity DataIC50: 3.48E+4nMAssay Description:Transcriptional activity at human androgen receptor BF3 site stably transfected in eGFP-expressing human LNCAP cells after 5 days by fluorometric ana...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)