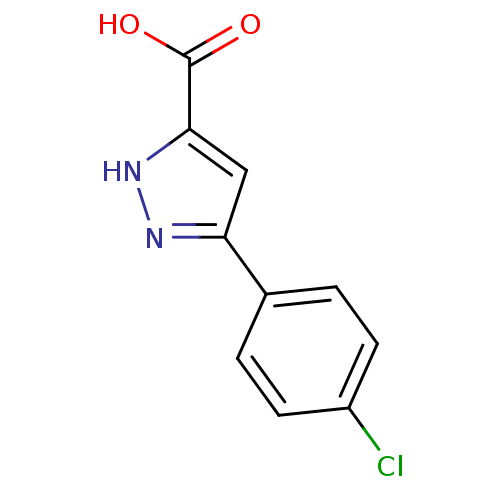
BDBM44423 3-(4-chlorophenyl)-1H-pyrazole-5-carboxylic acid::CHEMBL129261::MLS000113852::SMR000109742::cid_738819
SMILES OC(=O)c1cc(n[nH]1)-c1ccc(Cl)cc1
InChI Key InChIKey=WYTQCLKZYRFUIQ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 44423
Found 3 hits for monomerid = 44423
TargetHydroxycarboxylic acid receptor 2(Rattus norvegicus)
Leiden/Amsterdam Center For Drug Research
Curated by ChEMBL
Leiden/Amsterdam Center For Drug Research
Curated by ChEMBL
Affinity DataKi: 1.07E+5nMAssay Description:Inhibition of [3H]-nicotinic acid (20 nM) binding to nicotinic acid receptor in rat spleen membrane.More data for this Ligand-Target Pair
TargetAlkaline phosphatase, tissue-nonspecific isozyme(Homo sapiens (Human))
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 2.67E+3nMAssay Description:Sanford-Burnham Center for Chemical Genomics (SBCCG) Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) NIH Molecular Libraries Screen...More data for this Ligand-Target Pair
TargetAlkaline phosphatase, tissue-nonspecific isozyme(Homo sapiens (Human))
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 6.14E+3nMAssay Description:Sanford-Burnham Center for Chemical Genomics (SBCCG) Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) NIH Molecular Libraries Screen...More data for this Ligand-Target Pair