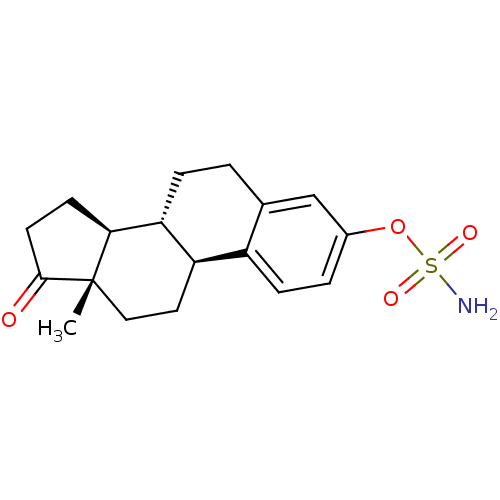
BDBM50134329 CHEMBL122708::Sulfamic acid (11R,12S,15S,16S)-13-methyl-17-oxo-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthren-3-yl ester::Sulfamic acid (8R,9S,13S,14S)-13-methyl-17-oxo-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthren-3-yl ester::estra-1,3,5(10)-trien-17-one-3-sulphamate::estrone3-O-sulfamate
SMILES C[C@]12CC[C@@H]3c4ccc(cc4CC[C@H]3[C@@H]1CCC2=O)OS(=O)(=O)N
InChI Key InChIKey=RVKFQAJIXCZXQY-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 71 hits for monomerid = 50134329
Found 71 hits for monomerid = 50134329
Affinity DataIC50: 0.0650nMAssay Description:Inhibitory concentration required to inhibit the enzyme estrone sulfatase was determinedMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0650nMAssay Description:Inhibition of STS in human MCF7 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0650nMAssay Description:Tested for the inhibitory activity against Estrone SulfataseMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0650nMAssay Description:Inhibition of STS in human MCF7 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0650nMAssay Description:Inhibitory activity against estrone sulfatase enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0650nMAssay Description:Inhibition of STS in human MCF7 cells assessed as reduction in [3H]estradiol and [3H]estrone formation using [3H]estrone sulfate as substrate incubat...More data for this Ligand-Target Pair
Affinity DataIC50: 0.830nMAssay Description:Inhibition of steroid sulfatase in human MCF7 cells using [3H]E1S as substrate after 20 hrs by scintillation spectrometryMore data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:Inhibition of human placental estrone sulfatase expressed in HEK293 cells using [3H]E1S as substrate incubated for 2 hrs by liquid scintillation coun...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:Inhibition of human placental steroid sulfatase expressed in HEK293 cells using [3H] E1S as substrate after 2 hrs by liquid scintillation counting me...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Inhibition of estrone sulfatase (unknown origin) transfected in HEK293 cells using [3H]E1S as substrate incubated for 2 hrs by liquid scintillation c...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Compound was tested for its ability to inhibit human embryonal kidney cell derived steroid sulfatase activity in transforming [3H]E1S (estrone sulfat...More data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:Inhibition of estrone sulfatase in human JEG-3 cells using [3H]-estrone sulphate as substrate incubated for 4 hrs by liquid scintillation counting me...More data for this Ligand-Target Pair
Affinity DataIC50: 5.60nMAssay Description:Compound was tested for its ability to inhibit the transformation of [C14]-DHEAS to DHEA by steroid sulfatase derived from human embryonal kidney cel...More data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of steroid sulfatase (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Compound was tested for inhibition of human carbonic anhydrase (hCA II)More data for this Ligand-Target Pair
Affinity DataIC50: 9.10nMAssay Description:Inhibition of placental microsomal estrone sulfatase (unknown origin) using [6,7-3H]E1S as substrate incubated for 1 hr by scintillation spectrometri...More data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Inhibition against human carbonic anhydrase IIMore data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Inhibition of human carbonic anhydrase IIMore data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Inhibition of recombinant human cytosolic isozyme CA IIMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibitory activity of compound against human carbonic anhydrase IIMore data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Inhibition of steroid sulfatase in human placental microsomes using [3H]E1S as substrate after 30 mins by scintillation spectrometryMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibitory activity against human steroid sulfatase over-expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibitory concentration against steroid sulfatase in placental microsomesMore data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:Inhibition of human carbonic anhydrase 2More data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:Inhibition of placental microsomal estrone sulfatase (unknown origin) using [3H]-estrone sulphate as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibitory activity against Steroid sulfatase expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Inhibitory concentration against catalytic domain of human cloned carbonic anhydrase IX.More data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Inhibition against human carbonic anhydrase IXMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibitory concentration against human steroid sulfatase expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Inhibition of cloned human transmembrane, tumor-associated isozyme CA IXMore data for this Ligand-Target Pair
Affinity DataKi: 37nMAssay Description:Inhibition of recombinant human cytosolic isozyme CA IMore data for this Ligand-Target Pair
Affinity DataKi: 37nMAssay Description:Inhibition of human recombinant carbonic anhydrase IMore data for this Ligand-Target Pair
Affinity DataKi: 37nMAssay Description:Inhibition against human carbonic anhydrase IMore data for this Ligand-Target Pair
Affinity DataIC50: 37nMAssay Description:Inhibitory activity of compound against human carbonic anhydrase IMore data for this Ligand-Target Pair
Affinity DataIC50: 42nMAssay Description:Inhibition of human CA2More data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition of purified human steroid sulfataseMore data for this Ligand-Target Pair
Affinity DataIC50: 56nMAssay Description:Inhibition of steroid sulfataseMore data for this Ligand-Target Pair
Affinity DataIC50: 56nMAssay Description:Inhibition of human CA2 by colorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 56nMAssay Description:Inhibition of human steroid sulfatase compared to EMATEMore data for this Ligand-Target Pair
Affinity DataIC50: 56nMAssay Description:Inhibition of human purified steroid sulfataseMore data for this Ligand-Target Pair
Affinity DataIC50: 56nMAssay Description:Inhibitory concentration against human steroid sulfataseMore data for this Ligand-Target Pair
Affinity DataIC50: 56nMAssay Description:Inhibitory activity against purified human Steroid sulfataseMore data for this Ligand-Target Pair
Affinity DataIC50: 56nMAssay Description:Inhibitory activity against purified human steroid sulfatase (STS)More data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Inhibition of human placental microsomal estrone sulfatase using 4-methylumbelliferyl sulfate as substrate incubated for 1 hr by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:Inhibition of STS activity in human placental microsomeMore data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:Tested for inhibition of estrone sulfatase in placental microsomal preparation (100000 g pellet) using substrate concentration of 20 uMMore data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:Inhibitory activity againist Estrone sulfatase from MCF-7 cells (placental microsomes)More data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:Inhibition of Estrone sulfatase in human placental microsomeMore data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:Inhibition of human placental microsomal estrone sulfatase using [3H]E1S as substrate incubated for 20 mins by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 93nMAssay Description:In vitro inhibition of estrone sulfatase in placental microsomesMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)