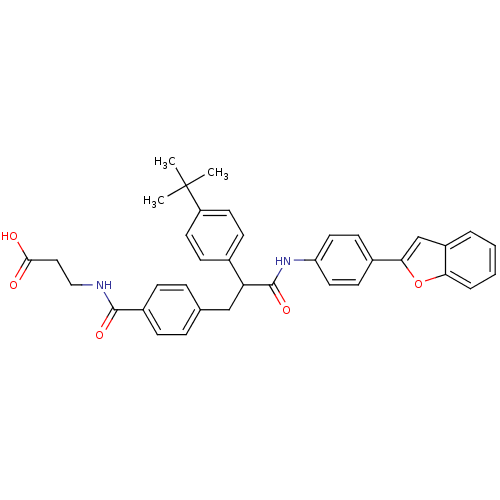
BDBM50144008 3-{4-[2-(4-Benzofuran-2-yl-phenylcarbamoyl)-2-(4-tert-butyl-phenyl)-ethyl]-benzoylamino}-propionic acid::CHEMBL305151
SMILES CC(C)(C)c1ccc(cc1)C(Cc1ccc(cc1)C(=O)NCCC(O)=O)C(=O)Nc1ccc(cc1)-c1cc2ccccc2o1
InChI Key InChIKey=GQPPIAAXSZCKMB-UHFFFAOYSA-N
Data 3 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50144008
Found 3 hits for monomerid = 50144008
Affinity DataKi: 4nMAssay Description:In vitro binding affinity against human glucagon receptor (h-GlucR) was determinedMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 29nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 69nMAssay Description:In vitro inhibitory activity against glucagon induced monkey adenylate cyclaseMore data for this Ligand-Target Pair