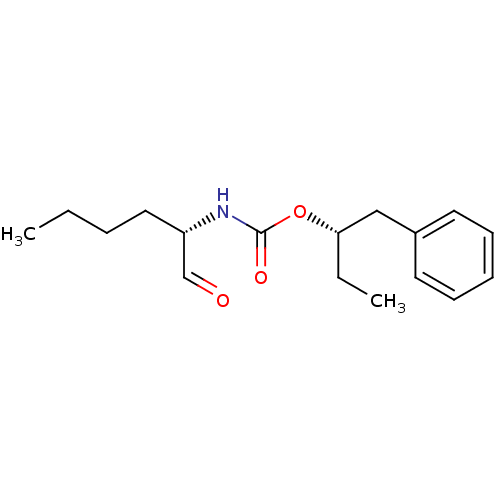
BDBM50148310 ((S)-1-Formyl-pentyl)-carbamic acid (S)-1-benzyl-propyl ester::CHEMBL114462
SMILES CCCC[C@H](NC(=O)O[C@@H](CC)Cc1ccccc1)C=O
InChI Key InChIKey=QQPGHYGBHVMSNJ-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50148310
Found 4 hits for monomerid = 50148310
Affinity DataIC50: 0.130nMAssay Description:Inhibitory concentration against human cathepsin K using 10 uM Cbz-Phe-Arg-AMCMore data for this Ligand-Target Pair
Affinity DataIC50: 0.130nMAssay Description:Inhibitory concentration against recombinant human cathepsin K determined in a fluorescence assay using 10 uM Cbz-Phe-Arg-AMC as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Inhibitory concentration against recombinant human cathepsin L was determined in a fluorescence assay using 5 uM Cbz-Phe-Arg-AMC as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 72nMAssay Description:Inhibitory concentration against recombinant human cathepsin B was determined in a fluorescence assay using 10 uM Cbz-Phe-Arg-AMC as substrateMore data for this Ligand-Target Pair