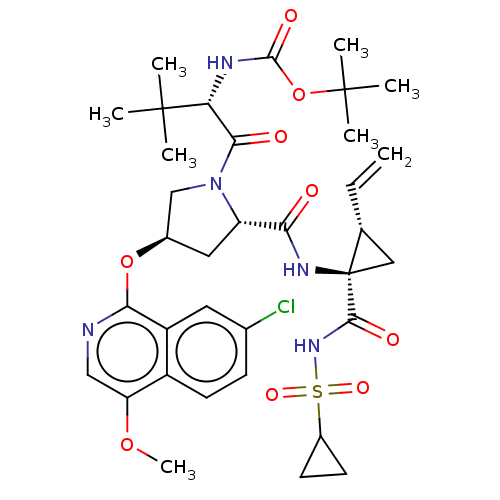
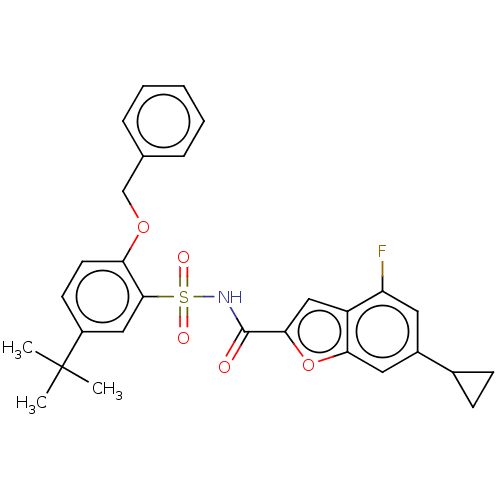
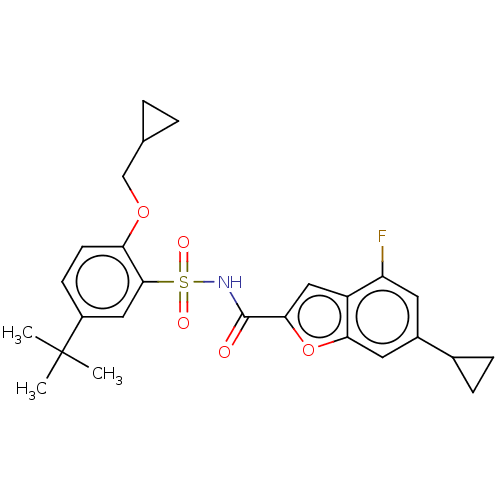
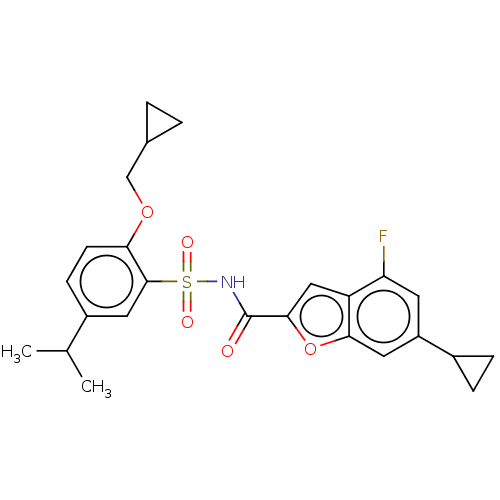
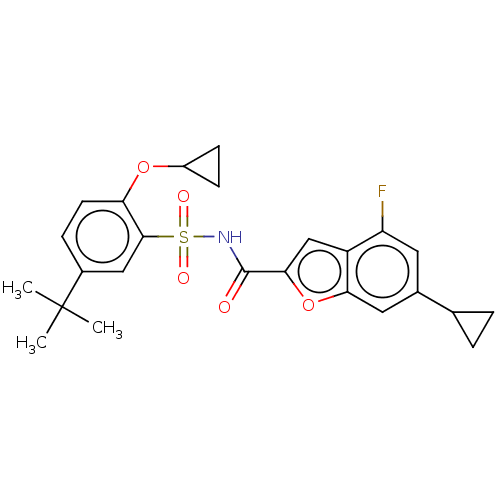
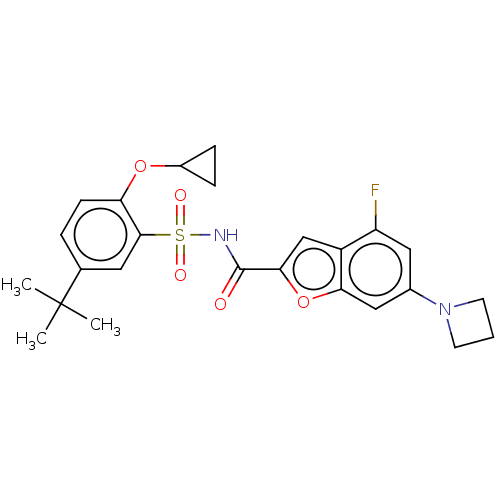
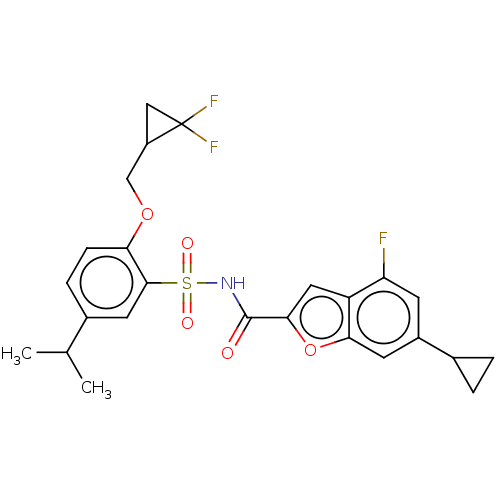
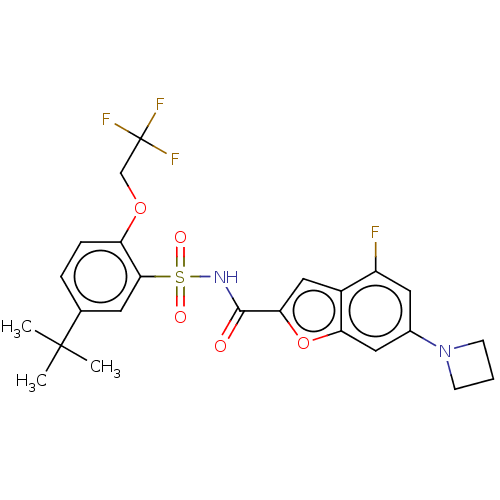
Affinity DataKi: 21nMAssay Description:Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligandMore data for this Ligand-Target Pair
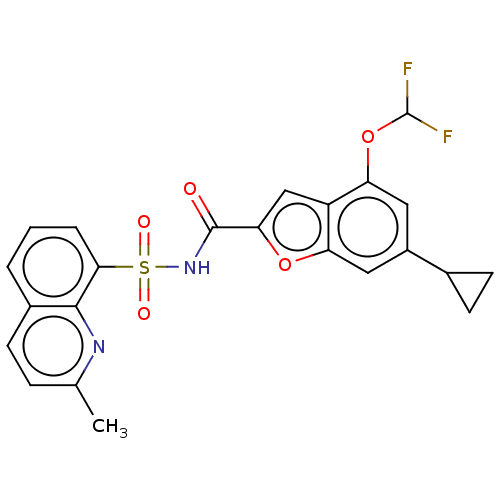
Affinity DataKi: 52nMAssay Description:Compound was evaluated for time-dependent inactivation of Ribonucleotide diphosphate reductase (RDPR) in E. coliMore data for this Ligand-Target Pair
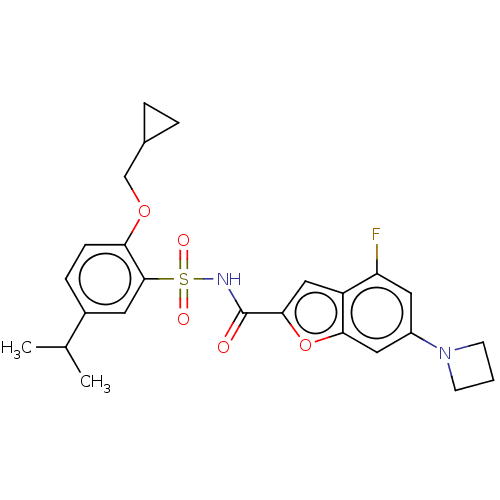
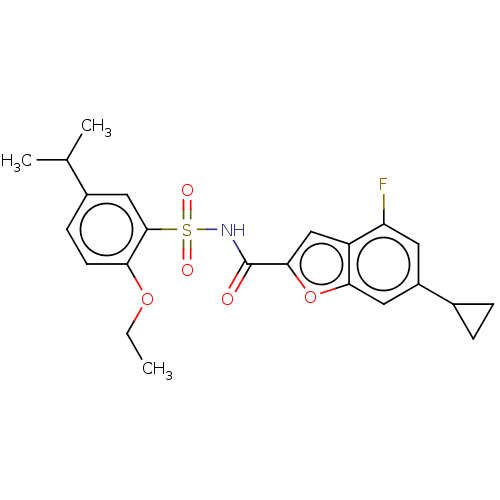
Affinity DataKi: 374nMAssay Description:Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligandMore data for this Ligand-Target Pair
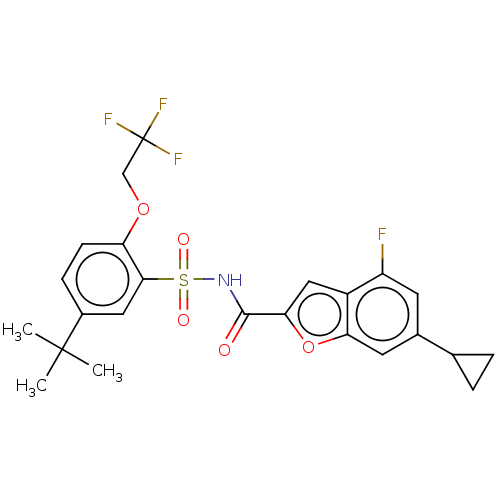
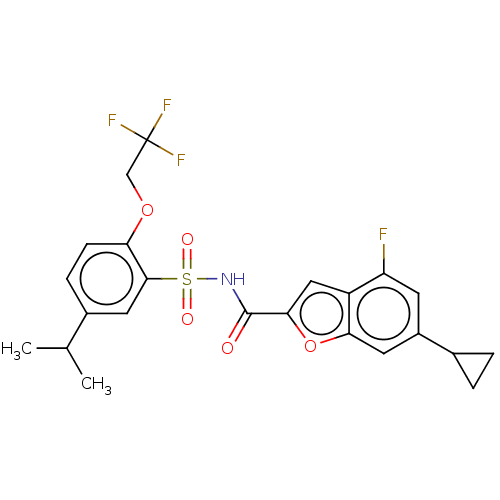
Affinity DataKi: 505nMAssay Description:Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligandMore data for this Ligand-Target Pair
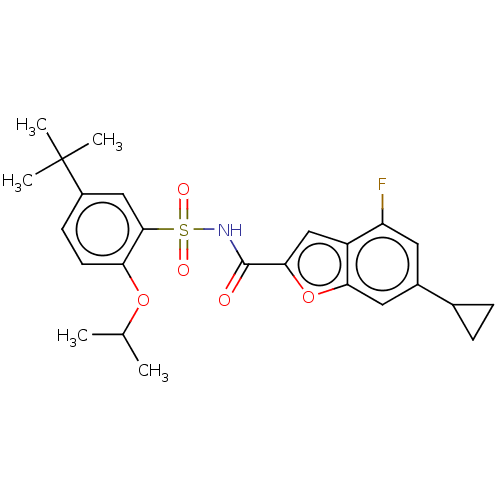
Affinity DataKi: >1.00E+3nMAssay Description:Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligandMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligandMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligandMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Binding affinity against Opioid receptor mu 1 using [3H]-etorphine as a radioligandMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligandMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligandMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 0.810nMAssay Description:Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR...More data for this Ligand-Target Pair
TargetGenome polyprotein/Non-structural protein 4A(Hepatitis C virus)
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
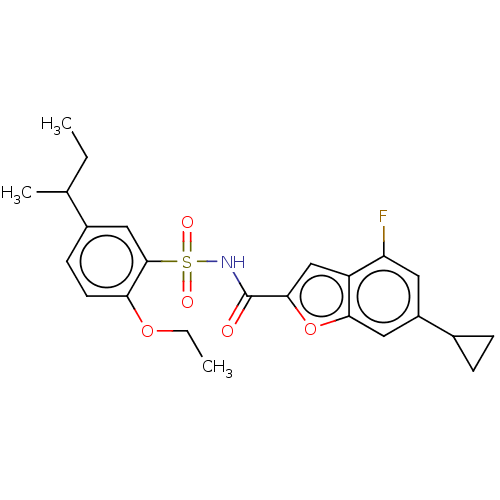
Affinity DataIC50: 1nMAssay Description:Inhibition of recombinant full length HCV genotype 1a NS3/4A protease (1027 to 1711 residues) expressed in Escherichia coli strain BL21 (DE3) using R...More data for this Ligand-Target Pair
TargetGenome polyprotein/Non-structural protein 4A(Hepatitis C virus)
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
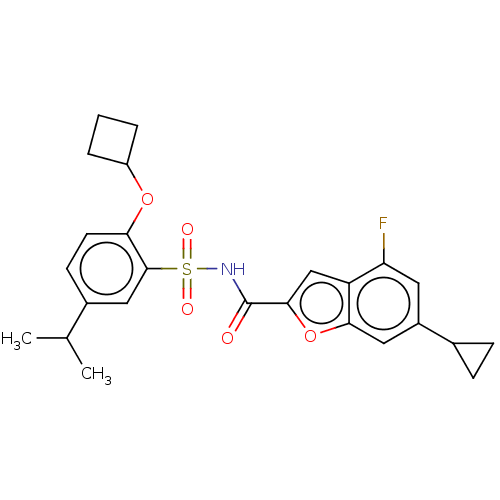
Affinity DataIC50: 1nMAssay Description:Inhibition of recombinant full length HCV genotype 1a NS3/4A protease (1027 to 1711 residues) expressed in Escherichia coli strain BL21 (DE3) using R...More data for this Ligand-Target Pair
Affinity DataIC50: 1.41nMAssay Description:Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR...More data for this Ligand-Target Pair
Affinity DataIC50: 1.42nMAssay Description:Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR...More data for this Ligand-Target Pair
Affinity DataIC50: 1.79nMAssay Description:Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR...More data for this Ligand-Target Pair
Affinity DataIC50: 1.84nMAssay Description: Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F...More data for this Ligand-Target Pair
Affinity DataIC50: 1.86nMAssay Description:Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR...More data for this Ligand-Target Pair
Affinity DataIC50: 2.09nMAssay Description: Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F...More data for this Ligand-Target Pair
Affinity DataIC50: 2.46nMAssay Description: Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F...More data for this Ligand-Target Pair
Affinity DataIC50: 2.57nMAssay Description:Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR...More data for this Ligand-Target Pair
Affinity DataIC50: 2.67nMAssay Description: Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F...More data for this Ligand-Target Pair
Affinity DataIC50: 2.92nMAssay Description: Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F...More data for this Ligand-Target Pair
Affinity DataIC50: 3.01nMAssay Description: Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F...More data for this Ligand-Target Pair
Affinity DataIC50: 3.31nMAssay Description: Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F...More data for this Ligand-Target Pair
Affinity DataIC50: 3.58nMAssay Description: Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F...More data for this Ligand-Target Pair
Affinity DataIC50: 3.72nMAssay Description:Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR...More data for this Ligand-Target Pair
Affinity DataIC50: 3.72nMAssay Description:Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR...More data for this Ligand-Target Pair
Affinity DataIC50: 3.91nMAssay Description:Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR...More data for this Ligand-Target Pair
Affinity DataIC50: 4.15nMAssay Description:Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR...More data for this Ligand-Target Pair
Affinity DataIC50: 4.16nMAssay Description: Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F...More data for this Ligand-Target Pair
Affinity DataIC50: 4.19nMAssay Description:Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR...More data for this Ligand-Target Pair
Affinity DataIC50: 4.22nMAssay Description:Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR...More data for this Ligand-Target Pair
Affinity DataIC50: 4.25nMAssay Description:Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR...More data for this Ligand-Target Pair
Affinity DataIC50: 4.33nMAssay Description: Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F...More data for this Ligand-Target Pair
Affinity DataIC50: 4.47nMAssay Description:Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR...More data for this Ligand-Target Pair
Affinity DataIC50: 4.48nMAssay Description:Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR...More data for this Ligand-Target Pair
Affinity DataIC50: 4.5nMAssay Description: Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F...More data for this Ligand-Target Pair
Affinity DataIC50: 4.53nMAssay Description:Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR...More data for this Ligand-Target Pair
Affinity DataIC50: 4.78nMAssay Description: Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F...More data for this Ligand-Target Pair
Affinity DataIC50: 4.79nMAssay Description:Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR...More data for this Ligand-Target Pair
Affinity DataIC50: 4.79nMAssay Description: Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F...More data for this Ligand-Target Pair
Affinity DataIC50: 4.99nMAssay Description: Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F...More data for this Ligand-Target Pair
TargetGenome polyprotein/Non-structural protein 4A(Hepatitis C virus)
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
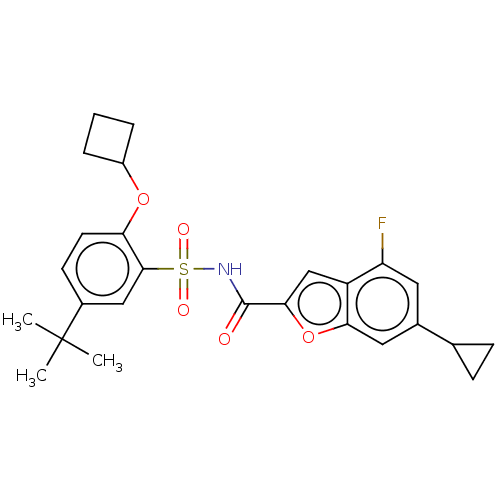
Affinity DataIC50: 5nMAssay Description:Inhibition of recombinant full length HCV genotype 1a NS3/4A protease (1027 to 1711 residues) expressed in Escherichia coli strain BL21 (DE3) using R...More data for this Ligand-Target Pair
Affinity DataIC50: 5.07nMAssay Description:Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR...More data for this Ligand-Target Pair
Affinity DataIC50: 5.20nMAssay Description:Binding affinity against opioid receptor kappa using [3H]U-69,593 as a radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 5.40nMAssay Description:Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-FR...More data for this Ligand-Target Pair
Affinity DataIC50: 5.40nMAssay Description:Binding affinity against Opioid receptor kappa 1 using [3H]U-69,593 as a radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 5.55nMAssay Description: Kat6a inhibitory activities of the compounds described in the present invention were quantified using a Fluorescence Resonance Energy Transfer (TR-F...More data for this Ligand-Target Pair

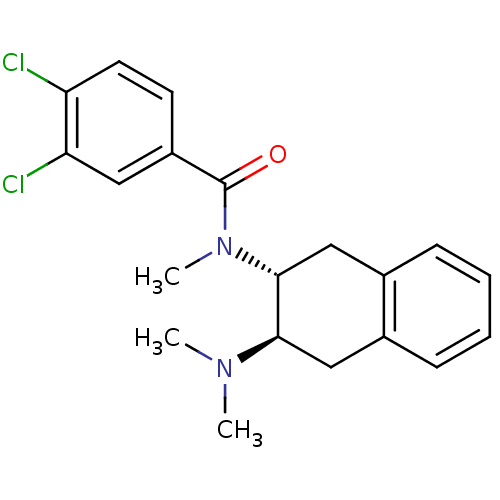

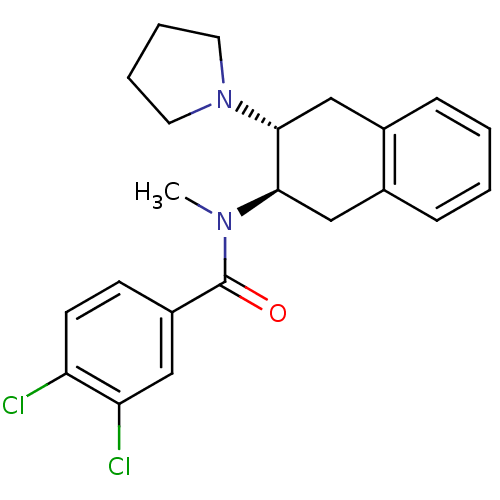

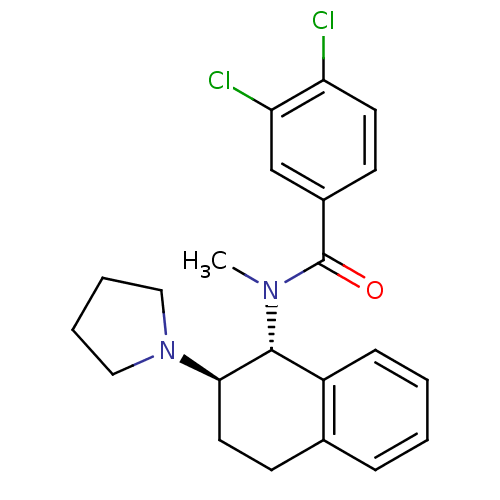

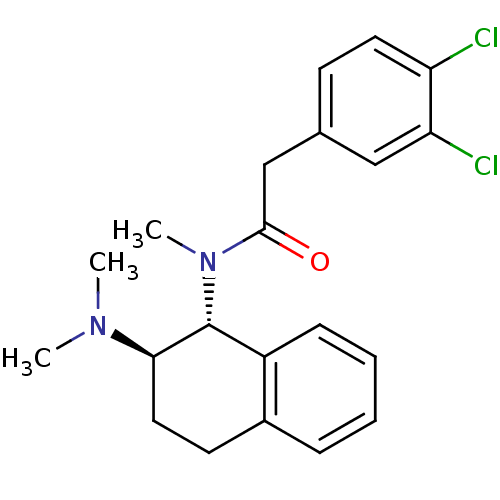
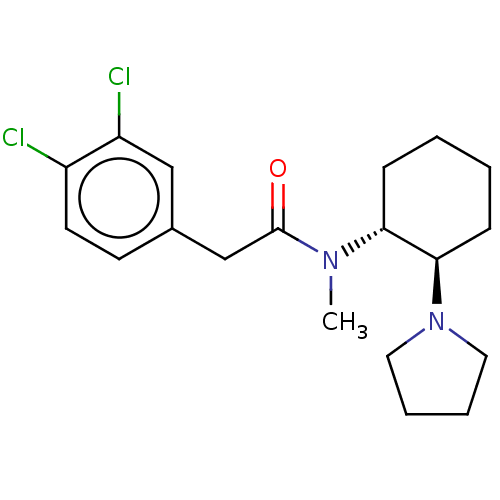
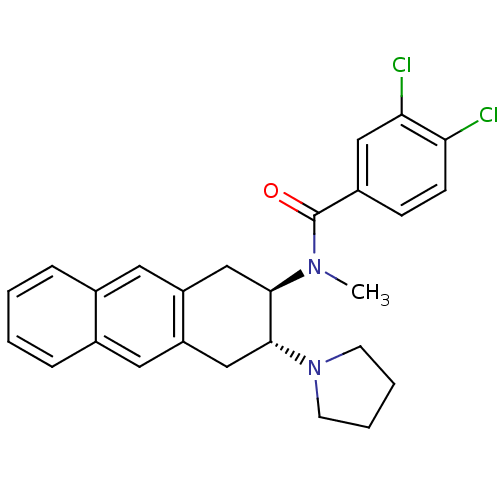
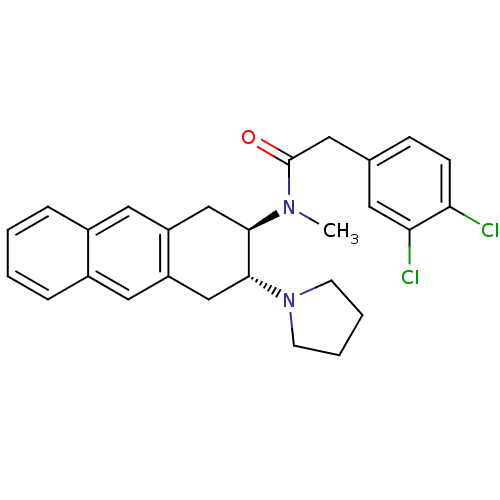



 3D Structure (crystal)
3D Structure (crystal)