Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Apelin receptor
Ligand
BDBM67390
Substrate
n/a
Meas. Tech.
Dose Response confirmation of uHTS hits from a small molecule antagonists of the APJ receptor via a luminescent beta-arrestin assay
IC50
24400±n/a nM
Citation
 PubChem, PC Dose Response confirmation of uHTS hits from a small molecule antagonists of the APJ receptor via a luminescent beta-arrestin assay PubChem Bioassay (2010)[AID]
PubChem, PC Dose Response confirmation of uHTS hits from a small molecule antagonists of the APJ receptor via a luminescent beta-arrestin assay PubChem Bioassay (2010)[AID] More Info.:
Target
Name:
Apelin receptor
Synonyms:
AGTRL1 | APJ | APJ_HUMAN | APLNR | Angiotensin receptor-like 1 | Apelin receptor | Apelin receptor (APJ) | G-protein coupled receptor APJ | G-protein coupled receptor HG11
Type:
Enzyme Catalytic Domain
Mol. Mass.:
42664.06
Organism:
Homo sapiens (Human)
Description:
P35414
Residue:
380
Sequence:
MEEGGDFDNYYGADNQSECEYTDWKSSGALIPAIYMLVFLLGTTGNGLVLWTVFRSSREKRRSADIFIASLAVADLTFVVTLPLWATYTYRDYDWPFGTFFCKLSSYLIFVNMYASVFCLTGLSFDRYLAIVRPVANARLRLRVSGAVATAVLWVLAALLAMPVMVLRTTGDLENTTKVQCYMDYSMVATVSSEWAWEVGLGVSSTTVGFVVPFTIMLTCYFFIAQTIAGHFRKERIEGLRKRRRLLSIIVVLVVTFALCWMPYHLVKTLYMLGSLLHWPCDFDLFLMNIFPYCTCISYVNSCLNPFLYAFFDPRFRQACTSMLCCGQSRCAGTSHSSSGEKSASYSSGHSQGPGPNMGKGGEQMHEKSIPYSQETLVVD
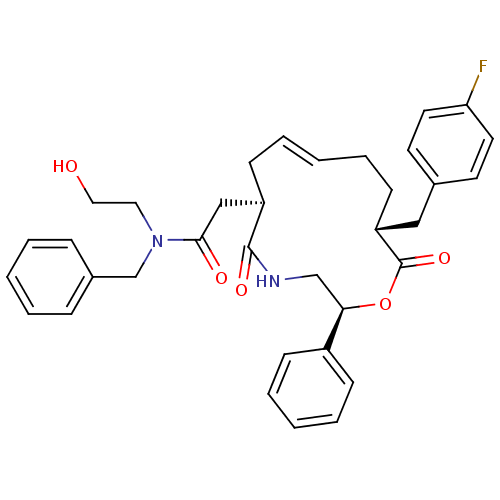
Inhibitor
Name:
BDBM67390
Synonyms:
2-[(2S,6R,8E,12R)-12-[(4-fluorophenyl)methyl]-5,13-bis(oxidanylidene)-2-phenyl-1-oxa-4-azacyclotridec-8-en-6-yl]-N-(2-hydroxyethyl)-N-(phenylmethyl)ethanamide | 2-[(2S,6R,8E,12R)-12-[(4-fluorophenyl)methyl]-5,13-dioxo-2-phenyl-1-oxa-4-azacyclotridec-8-en-6-yl]-N-(2-hydroxyethyl)-N-(phenylmethyl)acetamide | MLS002320220 | N-benzyl-2-[(2S,6R,8E,12R)-12-(4-fluorobenzyl)-5,13-diketo-2-phenyl-1-oxa-4-azacyclotridec-8-en-6-yl]-N-(2-hydroxyethyl)acetamide | N-benzyl-2-[(2S,6R,8E,12R)-12-[(4-fluorophenyl)methyl]-5,13-dioxo-2-phenyl-1-oxa-4-azacyclotridec-8-en-6-yl]-N-(2-hydroxyethyl)acetamide | SMR001337845 | cid_44201736
Type:
Small organic molecule
Emp. Form.:
C35H39FN2O5
Mol. Mass.:
586.693
SMILES:
OCCN(Cc1ccccc1)C(=O)C[C@H]1C\C=C\CC[C@H](Cc2ccc(F)cc2)C(=O)O[C@H](CNC1=O)c1ccccc1 |t:17|