Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Isoform 2 of Nuclear receptor corepressor 2 (TRAC-1)
Ligand
BDBM47286
Substrate
n/a
Meas. Tech.
Counterscreen for NR2E3 inverse agonists
IC50
>39839±n/a nM
Citation
 PubChem, PC Counterscreen for NR2E3 inverse agonists: TR-FRET-based biochemical high throughput dose response assay to identify inverse agonists of the interaction between peroxisome proliferator-activated receptor gamma (PPARg) and nuclear receptor co-repressor 2 (NCOR2) PubChem Bioassay (2010)[AID]
PubChem, PC Counterscreen for NR2E3 inverse agonists: TR-FRET-based biochemical high throughput dose response assay to identify inverse agonists of the interaction between peroxisome proliferator-activated receptor gamma (PPARg) and nuclear receptor co-repressor 2 (NCOR2) PubChem Bioassay (2010)[AID] More Info.:
Target
Name:
Isoform 2 of Nuclear receptor corepressor 2 (TRAC-1)
Synonyms:
CTG26 | NCOR2 | NCOR2_HUMAN | Nuclear receptor corepressor 2 (TRAC-1) | nuclear receptor corepressor 2 isoform 2
Type:
Enzyme Catalytic Domain
Mol. Mass.:
81401.89
Organism:
Homo sapiens (Human)
Description:
Q9Y618-2 gi_116256445
Residue:
766
Sequence:
MAQRADMLRGLSPRESSLALNYAAGPRGIIDLSQVPHLPVLVPPTPGTPATAMDRLAYLPTAPQPFSSRHSSSPLSPGGPTHLTKPTTTSSSERERDRDRERDRDREREKSILTSTTTVEHAPIWRPGTEQSSGSSGGGGGSSSRPASHSHAHQHSPISPRTQDALQQRPSVLHNTGMKGIITAVEPSTPTVLRSTSTSSPVRPAATFPPATHCPLGGTLDGVYPTLMEPVLLPKEAPRVARPERPRADTGHAFLAKPPARSGLEPASSPSKGSEPRPLVPPVSGHATIARTPAKNLAPHHASPDPPAPPASASDPHREKTQSKPFSIQELELRSLGYHGSSYSPEGVEPVSPVSSPSLTHDKGLPKHLEELDKSHLEGELRPKQPGPVKLGGEAAHLPHLRPLPESQPSSSPLLQTAPGVKGHQRVVTLAQHISEVITQDYTRHHPQQLSAPLPAPLYSFPGASCPVLDLRRPPSDLYLPPPDHGAPARGSPHSEGGKRSPEPNKTSVLGGGEDGIEPVSPPEGMTEPGHSRSAVYPLLYRDGEQTEPSRMGSKSPGNTSQPPAFFSKLTESNSAMVKSKKQEINKKLNTHNRNEPEYNISQPGTEIFNMPAITGTGLMTYRSQAVQEHASTNMGLEAIIRKALMGGGGKAKVSGRPSSRKAKSPAPGLASGDRPPSVSSVHSEGDCNRRTPLTNRVWEDRPSSAGSTPFPYNPLIMRLQAGVMASPPPPGLPAGSGPLAGPHHAWDEEPKPLLCSQYETLSDSE
Inhibitor
Name:
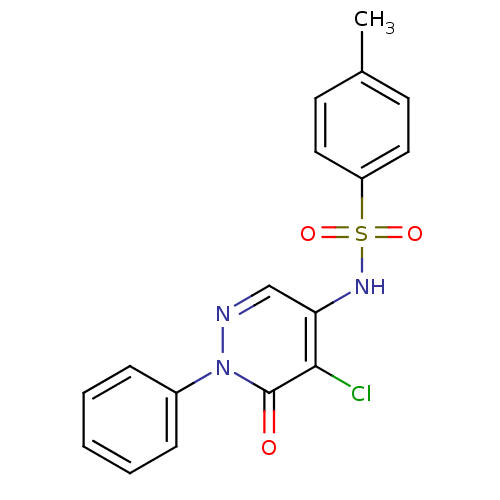
BDBM47286
Synonyms:
MLS000714107 | N-(5-chloranyl-6-oxidanylidene-1-phenyl-pyridazin-4-yl)-4-methyl-benzenesulfonamide | N-(5-chloro-6-keto-1-phenyl-pyridazin-4-yl)-4-methyl-benzenesulfonamide | N-(5-chloro-6-oxo-1-phenyl-4-pyridazinyl)-4-methylbenzenesulfonamide | N-(5-chloro-6-oxo-1-phenylpyridazin-4-yl)-4-methylbenzenesulfonamide | SMR000273587 | cid_1115929
Type:
Small organic molecule
Emp. Form.:
C17H14ClN3O3S
Mol. Mass.:
375.829
SMILES:
Cc1ccc(cc1)S(=O)(=O)Nc1cnn(-c2ccccc2)c(=O)c1Cl