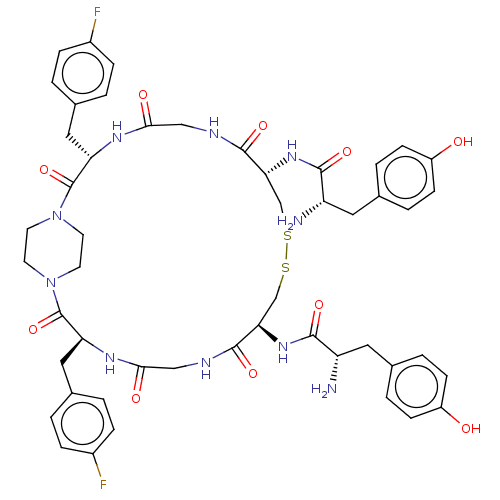
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Mu-type opioid receptor
Ligand
BDBM50526892
Substrate
n/a
Meas. Tech.
ChEMBL_1901658 (CHEMBL4403880)
Ki
84±n/a nM
Citation
 Stefanucci, A; Dimmito, MP; Macedonio, G; Ciarlo, L; Pieretti, S; Novellino, E; Lei, W; Barlow, D; Houseknecht, KL; Streicher, JM; Mollica, A Potent, Efficacious, and Stable Cyclic Opioid Peptides with Long Lasting Antinociceptive Effect after Peripheral Administration. J Med Chem 63:2673-2687 (2020) [PubMed] Article
Stefanucci, A; Dimmito, MP; Macedonio, G; Ciarlo, L; Pieretti, S; Novellino, E; Lei, W; Barlow, D; Houseknecht, KL; Streicher, JM; Mollica, A Potent, Efficacious, and Stable Cyclic Opioid Peptides with Long Lasting Antinociceptive Effect after Peripheral Administration. J Med Chem 63:2673-2687 (2020) [PubMed] Article More Info.:
Target
Name:
Mu-type opioid receptor
Synonyms:
M-OR-1 | MOP | MOR-1 | MOR1 | MUOR1 | Mu Opioid Receptor | Mu opiate receptor | OPIATE Mu | OPRM1 | OPRM_HUMAN | hMOP | mu-type opioid receptor isoform MOR-1
Type:
G Protein-Coupled Receptor (GPCR)
Mol. Mass.:
44789.51
Organism:
Homo sapiens (Human)
Description:
P35372
Residue:
400
Sequence:
MDSSAAPTNASNCTDALAYSSCSPAPSPGSWVNLSHLDGNLSDPCGPNRTDLGGRDSLCPPTGSPSMITAITIMALYSIVCVVGLFGNFLVMYVIVRYTKMKTATNIYIFNLALADALATSTLPFQSVNYLMGTWPFGTILCKIVISIDYYNMFTSIFTLCTMSVDRYIAVCHPVKALDFRTPRNAKIINVCNWILSSAIGLPVMFMATTKYRQGSIDCTLTFSHPTWYWENLLKICVFIFAFIMPVLIITVCYGLMILRLKSVRMLSGSKEKDRNLRRITRMVLVVVAVFIVCWTPIHIYVIIKALVTIPETTFQTVSWHFCIALGYTNSCLNPVLYAFLDENFKRCFREFCIPTSSNIEQQNSTRIRQNTRDHPSTANTVDRTNHQLENLEAETAPLP
Inhibitor
Name:
BDBM50526892
Synonyms:
CHEMBL4551481
Type:
Small organic molecule
Emp. Form.:
C54H60F8N10O14S2
Mol. Mass.:
1289.23
SMILES:
OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccc(F)cc2)C(=O)N2CCN(CC2)C(=O)[C@H](Cc2ccc(F)cc2)NC(=O)CNC1=O |r,wU:27.25,15.13,72.74,36.36,wD:53.53,32.31,(34.88,-40.7,;33.52,-39.92,;33.52,-38.35,;32.16,-40.7,;30.8,-39.92,;32.16,-42.27,;30.8,-41.48,;15.86,-46.31,;14.5,-45.53,;14.5,-43.96,;13.14,-46.31,;11.78,-45.53,;13.14,-47.88,;11.77,-47.09,;10.88,-39.84,;12.21,-39.06,;12.21,-37.52,;10.88,-36.76,;10.89,-35.22,;9.55,-34.45,;8.22,-35.22,;6.88,-34.45,;8.23,-36.77,;9.55,-37.52,;13.55,-39.84,;13.55,-41.38,;14.88,-39.06,;16.21,-39.84,;16.2,-41.41,;17.41,-42.61,;19.59,-44.11,;21.55,-44.45,;22.89,-43.66,;24.23,-44.42,;25.56,-43.65,;25.55,-42.11,;26.89,-44.41,;28.22,-43.64,;26.9,-45.95,;28.25,-46.73,;28.25,-48.29,;29.61,-49.06,;30.96,-48.28,;32.32,-49.05,;30.94,-46.71,;29.59,-45.94,;22.89,-42.12,;24.21,-41.34,;21.55,-41.36,;21.54,-39.82,;22.87,-39.05,;24.21,-39.81,;22.87,-37.5,;24.19,-36.73,;25.54,-37.5,;26.86,-36.72,;28.19,-37.49,;29.51,-36.71,;29.51,-35.17,;30.83,-34.39,;28.17,-34.41,;26.84,-35.19,;24.19,-35.19,;25.53,-34.41,;22.85,-34.43,;21.52,-35.2,;20.19,-34.44,;20.18,-32.9,;21.51,-32.13,;22.85,-32.89,;18.84,-32.14,;18.83,-30.59,;17.52,-32.91,;16.18,-32.15,;16.17,-30.61,;17.5,-29.84,;17.5,-28.31,;16.16,-27.54,;16.15,-26,;14.83,-28.33,;14.84,-29.86,;17.52,-34.45,;16.19,-35.23,;14.86,-34.46,;16.2,-36.77,;17.54,-37.52,;17.54,-39.06,;18.88,-39.83,)|