Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Endothelin-1 receptor
Ligand
BDBM50027796
Substrate
n/a
Meas. Tech.
ChEMBL_63504 (CHEMBL676594)
IC50
69000±n/a nM
Citation
 Wermuth, CG Selective optimization of side activities: another way for drug discovery. J Med Chem 47:1303-14 (2004) [PubMed] Article
Wermuth, CG Selective optimization of side activities: another way for drug discovery. J Med Chem 47:1303-14 (2004) [PubMed] Article More Info.:
Target
Name:
Endothelin-1 receptor
Synonyms:
EDNRA | EDNRA_HUMAN | ET-A | ETA | ETA-R | ETRA | Endothelin receptor type A | Endothelin receptor, ET-A/ET-B | hET-AR
Type:
Enzyme Catalytic Domain
Mol. Mass.:
48736.88
Organism:
Homo sapiens (Human)
Description:
P25101
Residue:
427
Sequence:
METLCLRASFWLALVGCVISDNPERYSTNLSNHVDDFTTFRGTELSFLVTTHQPTNLVLPSNGSMHNYCPQQTKITSAFKYINTVISCTIFIVGMVGNATLLRIIYQNKCMRNGPNALIASLALGDLIYVVIDLPINVFKLLAGRWPFDHNDFGVFLCKLFPFLQKSSVGITVLNLCALSVDRYRAVASWSRVQGIGIPLVTAIEIVSIWILSFILAIPEAIGFVMVPFEYRGEQHKTCMLNATSKFMEFYQDVKDWWLFGFYFCMPLVCTAIFYTLMTCEMLNRRNGSLRIALSEHLKQRREVAKTVFCLVVIFALCWFPLHLSRILKKTVYNEMDKNRCELLSFLLLMDYIGINLATMNSCINPIALYFVSKKFKNCFQSCLCCCCYQSKSLMTSVPMNGTSIQWKNHDQNNHNTDRSSHKDSMN
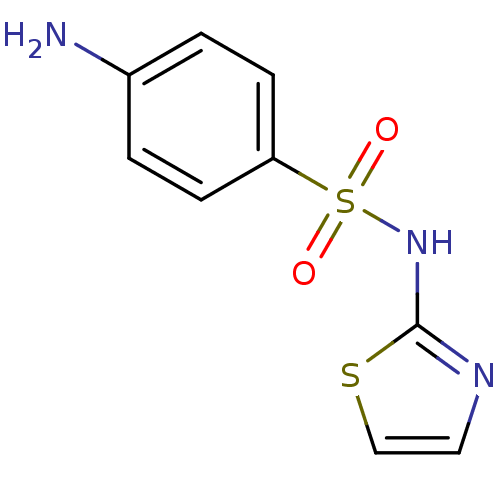
Inhibitor
Name:
BDBM50027796
Synonyms:
2-(Sulfanilylamino)thiazole | 2-(p-Aminobenzenesulfonamido)thiazole | 2-(p-Aminobenzenesulphonamido)thiazole | 2-Sulfanilamidothiazol | 2-Sulfanilamidothiazole | 2-Sulfonamidothiazole | 4-Amino-N-2-thiazolylbenzenesulfonamide | 4-amino-N-1,3-thiazol-2-ylbenzenesulfonamide | CHEMBL437 | N(1)-2-Thiazolylsulfanilamide | Sulfanilamidothiazole | Sulfathiazole | Sulphathiazole | cid_5340
Type:
Small organic molecule
Emp. Form.:
C9H9N3O2S2
Mol. Mass.:
255.317
SMILES:
Nc1ccc(cc1)S(=O)(=O)Nc1nccs1