Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
5-hydroxytryptamine receptor 2C
Ligand
BDBM139370
Substrate
n/a
Meas. Tech.
Receptor Selection and Amplification Technology
IC50
91±31 nM
Citation
 Vanover, KE; Weiner, DM; Makhay, M; Veinbergs, I; Gardell, LR; Lameh, J; Del Tredici, AL; Piu, F; Schiffer, HH; Ott, TR; Burstein, ES; Uldam, AK; Thygesen, MB; Schlienger, N; Andersson, CM; Son, TY; Harvey, SC; Powell, SB; Geyer, MA; Tolf, BR; Brann, MR; Davis, RE Pharmacological and behavioral profile of N-(4-fluorophenylmethyl)-N-(1-methylpiperidin-4-yl)-N'-(4-(2-methylpropyloxy)phenylmethyl) carbamide (2R,3R)-dihydroxybutanedioate (2:1) (ACP-103), a novel 5-hydroxytryptamine(2A) receptor inverse agonist. J Pharmacol Exp Ther 317:910-8 (2006) [PubMed] Article
Vanover, KE; Weiner, DM; Makhay, M; Veinbergs, I; Gardell, LR; Lameh, J; Del Tredici, AL; Piu, F; Schiffer, HH; Ott, TR; Burstein, ES; Uldam, AK; Thygesen, MB; Schlienger, N; Andersson, CM; Son, TY; Harvey, SC; Powell, SB; Geyer, MA; Tolf, BR; Brann, MR; Davis, RE Pharmacological and behavioral profile of N-(4-fluorophenylmethyl)-N-(1-methylpiperidin-4-yl)-N'-(4-(2-methylpropyloxy)phenylmethyl) carbamide (2R,3R)-dihydroxybutanedioate (2:1) (ACP-103), a novel 5-hydroxytryptamine(2A) receptor inverse agonist. J Pharmacol Exp Ther 317:910-8 (2006) [PubMed] Article More Info.:
Target
Name:
5-hydroxytryptamine receptor 2C
Synonyms:
5-HT-2C | 5-HT2C | 5-HT2C-INI | 5-HT2c VGI | 5-HTR2C | 5-hydroxytryptamine receptor 1C | 5-hydroxytryptamine receptor 2C (5-HT-2C) | 5-hydroxytryptamine receptor 2C (5HT-2C) | 5HT-1C | 5HT2C_HUMAN | HTR1C | HTR2C | Serotonin (5-HT3) receptor | Serotonin 2c (5-HT2c) receptor | Serotonin Receptor 2C
Type:
G Protein-Coupled Receptor (GPCR)
Mol. Mass.:
51836.79
Organism:
Homo sapiens (Human)
Description:
P28335
Residue:
458
Sequence:
MVNLRNAVHSFLVHLIGLLVWQCDISVSPVAAIVTDIFNTSDGGRFKFPDGVQNWPALSIVIIIIMTIGGNILVIMAVSMEKKLHNATNYFLMSLAIADMLVGLLVMPLSLLAILYDYVWPLPRYLCPVWISLDVLFSTASIMHLCAISLDRYVAIRNPIEHSRFNSRTKAIMKIAIVWAISIGVSVPIPVIGLRDEEKVFVNNTTCVLNDPNFVLIGSFVAFFIPLTIMVITYCLTIYVLRRQALMLLHGHTEEPPGLSLDFLKCCKRNTAEEENSANPNQDQNARRRKKKERRPRGTMQAINNERKASKVLGIVFFVFLIMWCPFFITNILSVLCEKSCNQKLMEKLLNVFVWIGYVCSGINPLVYTLFNKIYRRAFSNYLRCNYKVEKKPPVRQIPRVAATALSGRELNVNIYRHTNEPVIEKASDNEPGIEMQVENLELPVNPSSVVSERISSV
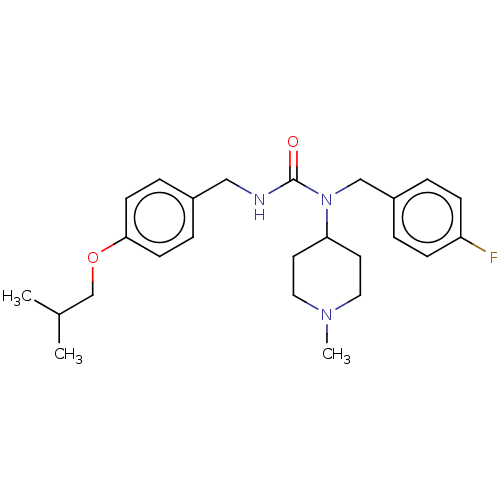
Inhibitor
Name:
BDBM139370
Synonyms:
ACP-103 | Nuplazid | Pimavanserin | Pimavanserin hydrochloride | Pimavanserin tartrate | US20230348421, Compound Pimavanserin | WO2023288027, Cmpd PIMA | bis(1-(4-fluorobenzyl)-1-(1-methylpiperidin-4-yl)-3-(4-(2-methylpropoxy)benzyl)urea) (2R,3R)-2,3-dihydroxybutanedioate
Type:
Small organic molecule
Emp. Form.:
C25H34FN3O2
Mol. Mass.:
427.5548
SMILES:
CC(C)COc1ccc(CNC(=O)N(Cc2ccc(F)cc2)C2CCN(C)CC2)cc1