Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Proto-oncogene tyrosine-protein kinase receptor Ret
Ligand
BDBM473675
Substrate
n/a
Meas. Tech.
Time-Resolved Fluorescence Transfer (FRET) Assay
IC50
6.90±n/a nM
Citation
 Jordan, AM; Newton, R; Waszkowycz, B; Sutton, JM; Hynd, G; Paoletta, S; Fordyce, EA Heterocyclic compounds as RET kinase inhibitors US Patent US10844067 Publication Date 11/24/2020
Jordan, AM; Newton, R; Waszkowycz, B; Sutton, JM; Hynd, G; Paoletta, S; Fordyce, EA Heterocyclic compounds as RET kinase inhibitors US Patent US10844067 Publication Date 11/24/2020 More Info.:
Target
Name:
Proto-oncogene tyrosine-protein kinase receptor Ret
Synonyms:
CDHF12 | CDHR16 | Cadherin family member 12 | PTC | Proto-oncogene c-Ret | RET | RET51 | RET_HUMAN | Tyrosine-protein kinase Ret (RET)
Type:
Protein
Mol. Mass.:
124318.29
Organism:
Homo sapiens (Human)
Description:
P07949
Residue:
1114
Sequence:
MAKATSGAAGLRLLLLLLLPLLGKVALGLYFSRDAYWEKLYVDQAAGTPLLYVHALRDAPEEVPSFRLGQHLYGTYRTRLHENNWICIQEDTGLLYLNRSLDHSSWEKLSVRNRGFPLLTVYLKVFLSPTSLREGECQWPGCARVYFSFFNTSFPACSSLKPRELCFPETRPSFRIRENRPPGTFHQFRLLPVQFLCPNISVAYRLLEGEGLPFRCAPDSLEVSTRWALDREQREKYELVAVCTVHAGAREEVVMVPFPVTVYDEDDSAPTFPAGVDTASAVVEFKRKEDTVVATLRVFDADVVPASGELVRRYTSTLLPGDTWAQQTFRVEHWPNETSVQANGSFVRATVHDYRLVLNRNLSISENRTMQLAVLVNDSDFQGPGAGVLLLHFNVSVLPVSLHLPSTYSLSVSRRARRFAQIGKVCVENCQAFSGINVQYKLHSSGANCSTLGVVTSAEDTSGILFVNDTKALRRPKCAELHYMVVATDQQTSRQAQAQLLVTVEGSYVAEEAGCPLSCAVSKRRLECEECGGLGSPTGRCEWRQGDGKGITRNFSTCSPSTKTCPDGHCDVVETQDINICPQDCLRGSIVGGHEPGEPRGIKAGYGTCNCFPEEEKCFCEPEDIQDPLCDELCRTVIAAAVLFSFIVSVLLSAFCIHCYHKFAHKPPISSAEMTFRRPAQAFPVSYSSSGARRPSLDSMENQVSVDAFKILEDPKWEFPRKNLVLGKTLGEGEFGKVVKATAFHLKGRAGYTTVAVKMLKENASPSELRDLLSEFNVLKQVNHPHVIKLYGACSQDGPLLLIVEYAKYGSLRGFLRESRKVGPGYLGSGGSRNSSSLDHPDERALTMGDLISFAWQISQGMQYLAEMKLVHRDLAARNILVAEGRKMKISDFGLSRDVYEEDSYVKRSQGRIPVKWMAIESLFDHIYTTQSDVWSFGVLLWEIVTLGGNPYPGIPPERLFNLLKTGHRMERPDNCSEEMYRLMLQCWKQEPDKRPVFADISKDLEKMMVKRRDYLDLAASTPSDSLIYDDGLSEEETPLVDCNNAPLPRALPSTWIENKLYGMSDPNWPGESPVPLTRADGTNTGFPRYPNDSVYANWMLSPSAAKLMDTFDS
Inhibitor
Name:
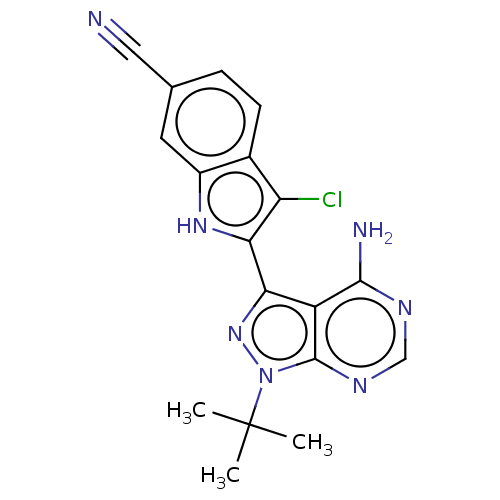
BDBM473675
Synonyms:
2-(4-Amino-1-(tert-butyl)-1H-pyrazolo[3,4- d]pyrimidin-3-yl)-3-chloro-1H-indole-6- carbonitrile | US10844067, Example 18 | US11661423, Example 18
Type:
Small organic molecule
Emp. Form.:
C18H16ClN7
Mol. Mass.:
365.82
SMILES:
CC(C)(C)n1nc(-c2[nH]c3cc(ccc3c2Cl)C#N)c2c(N)ncnc12