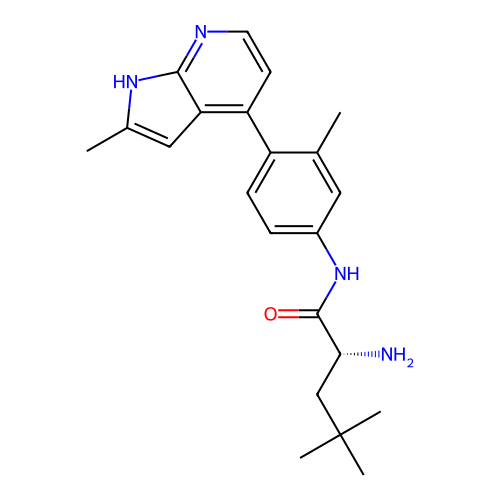
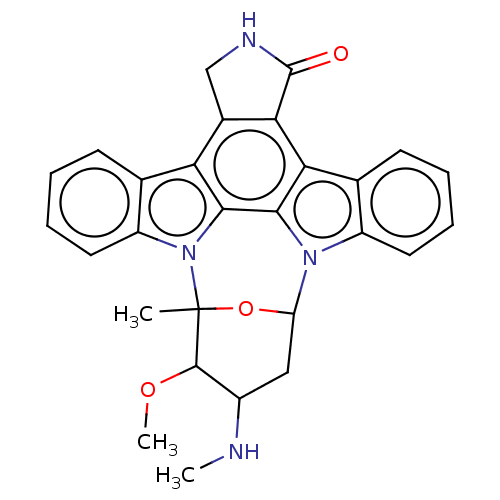
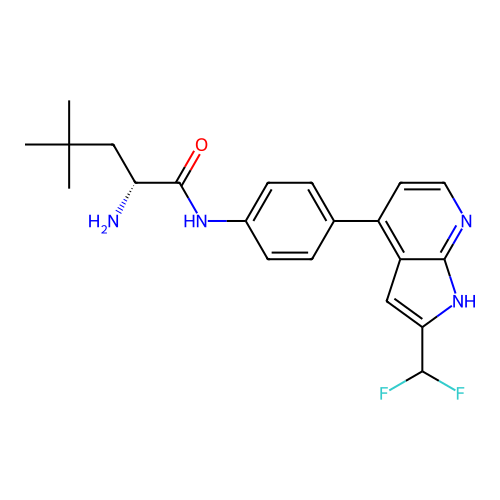
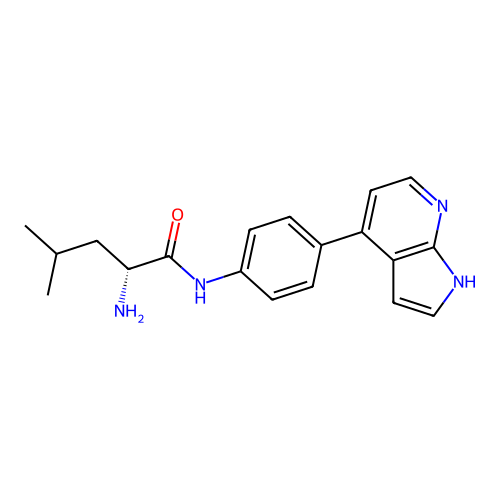
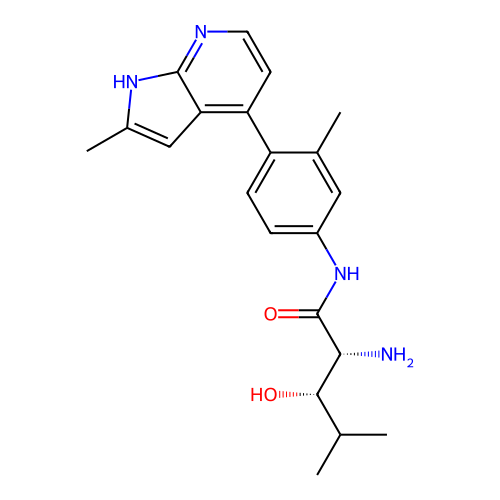
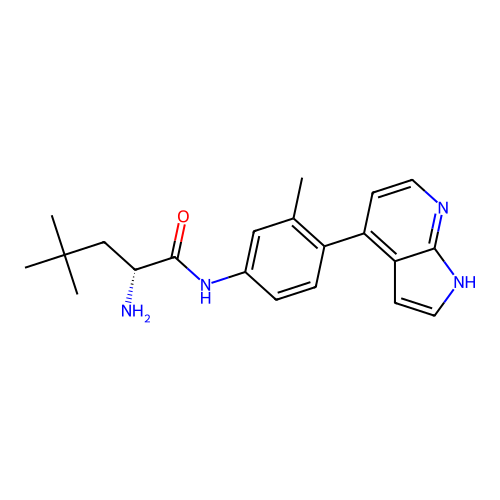
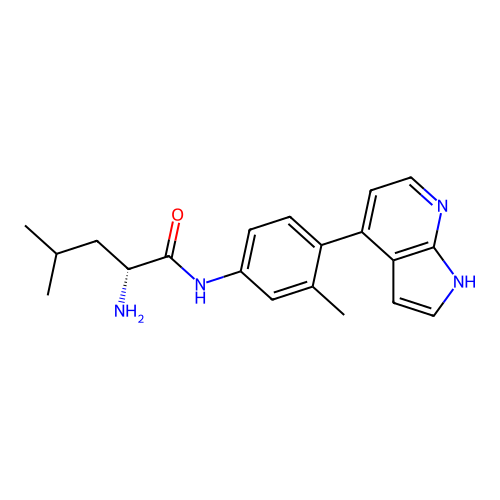
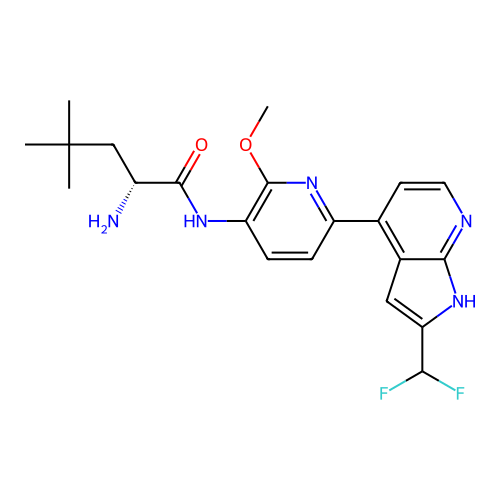
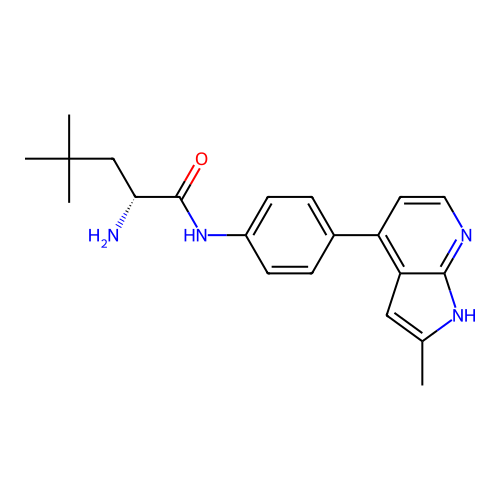
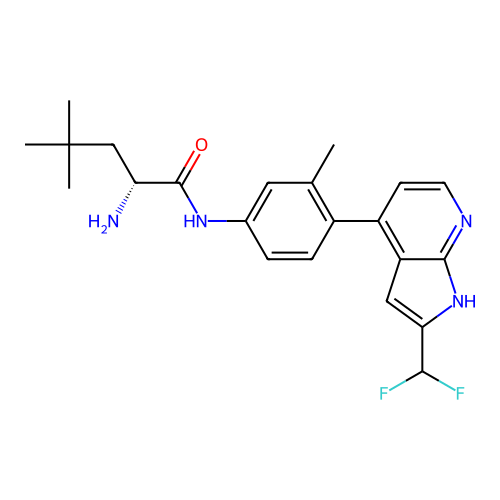
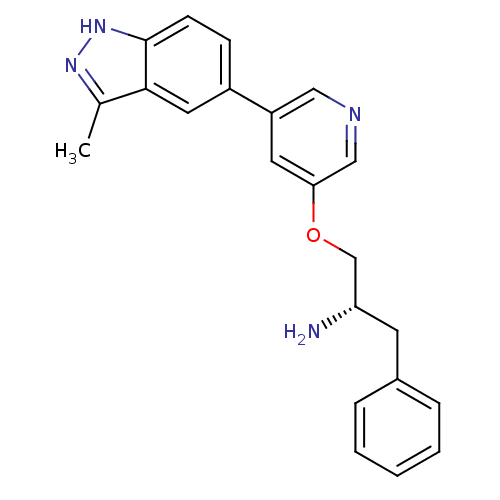
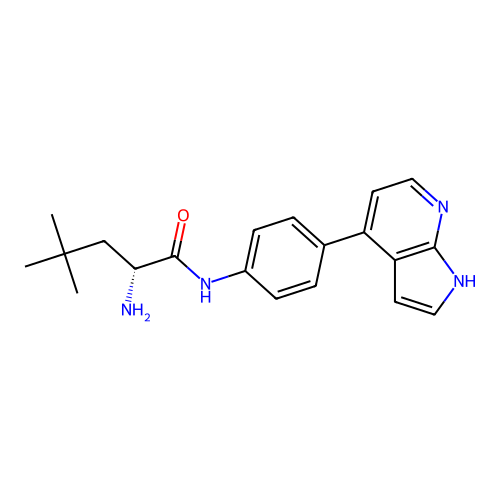
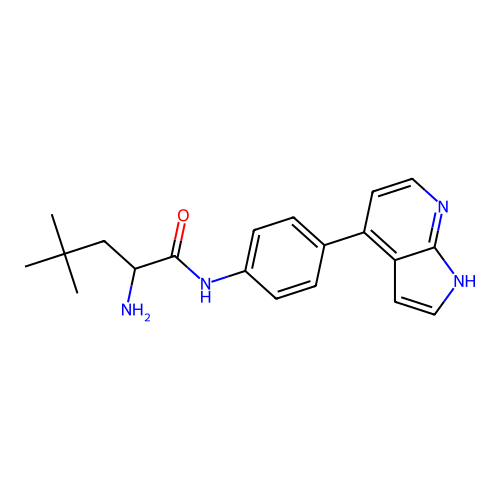
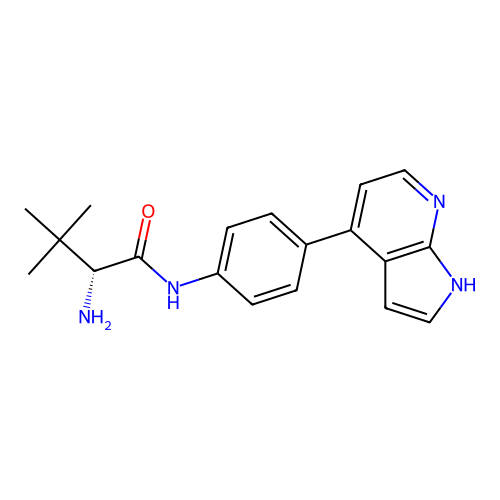
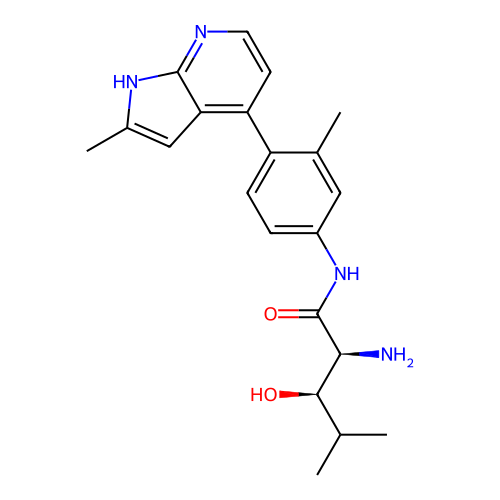
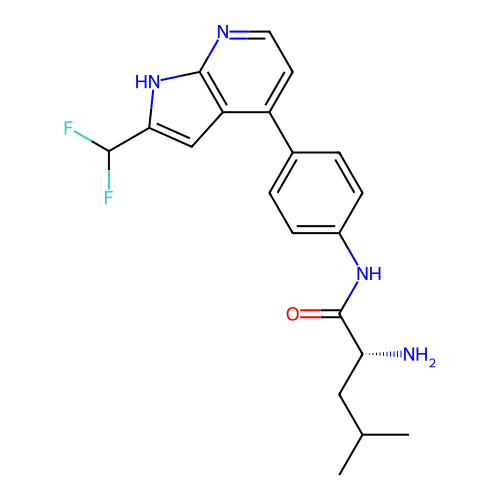
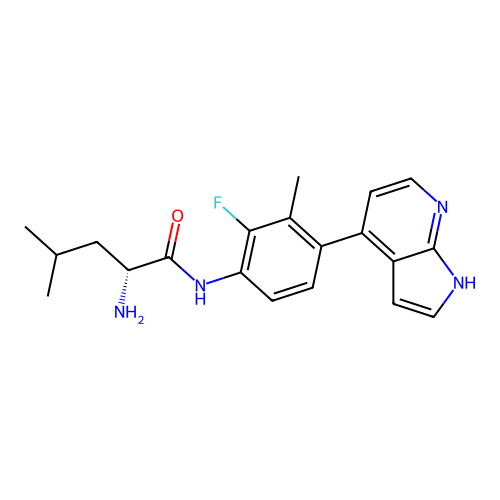
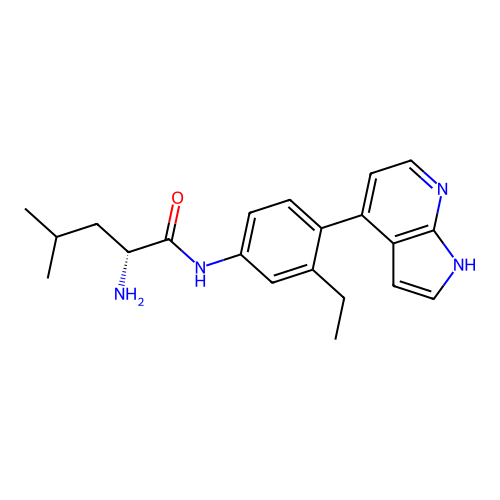
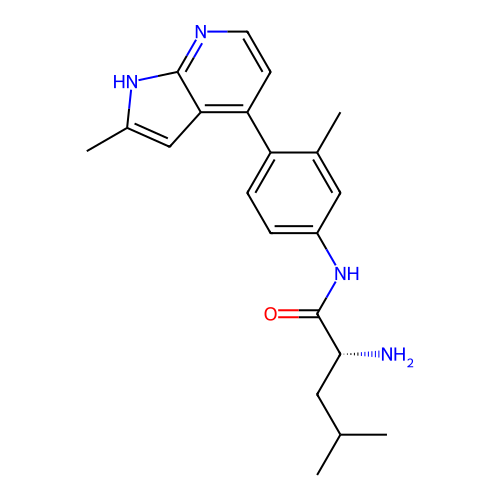
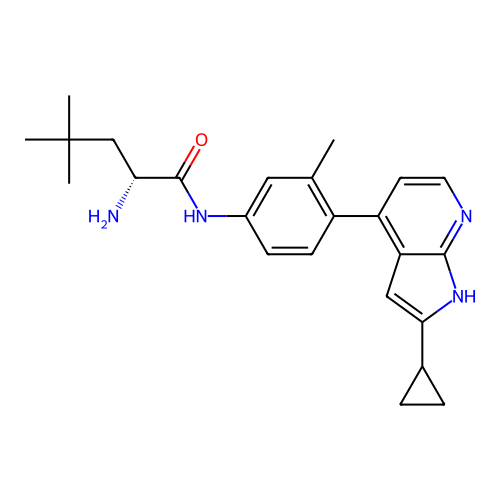
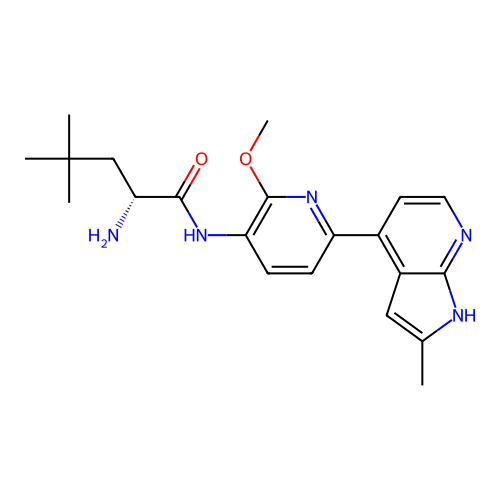
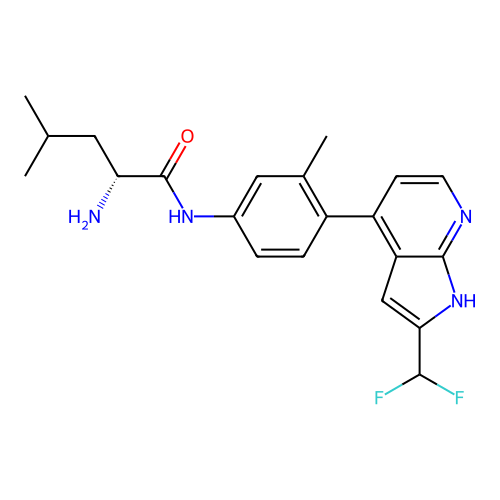
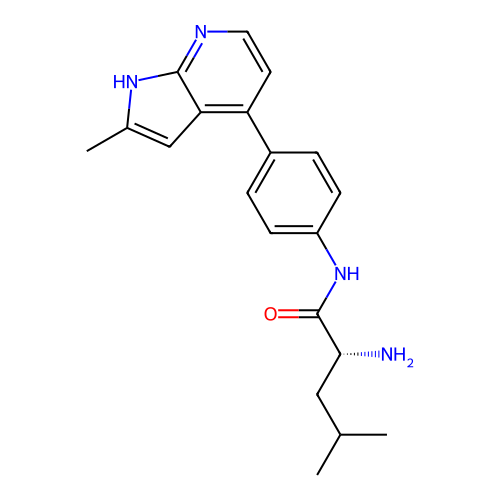
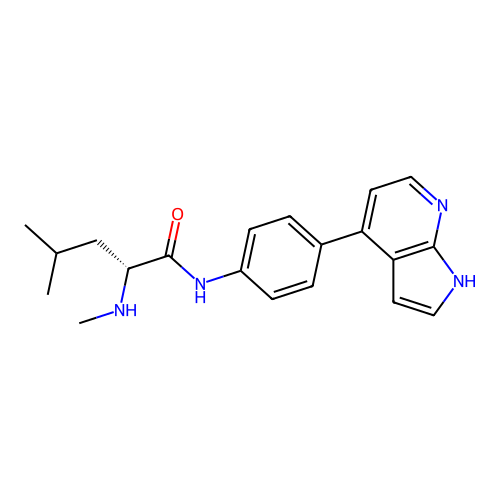
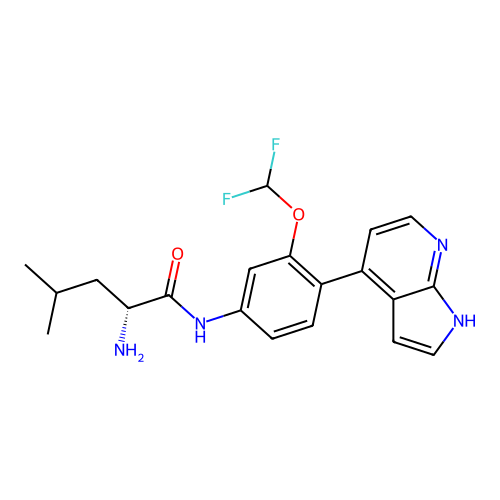
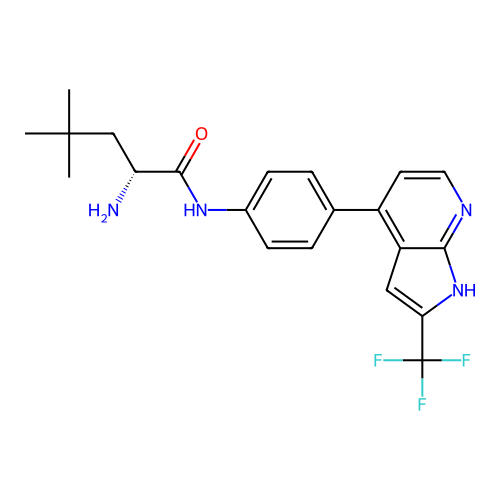
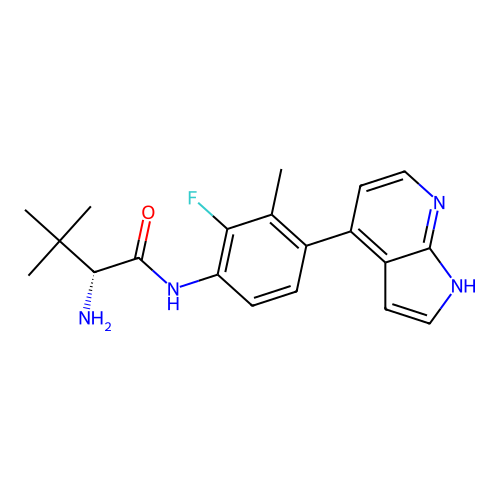
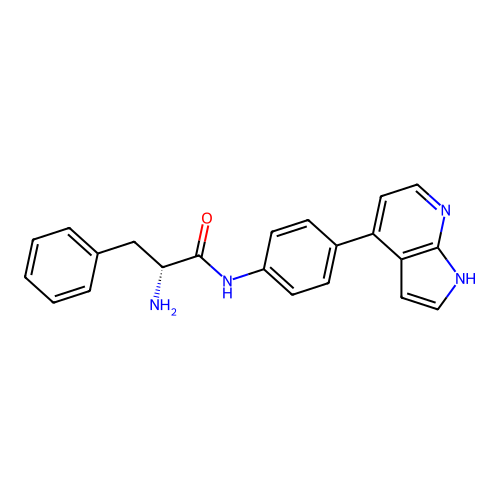
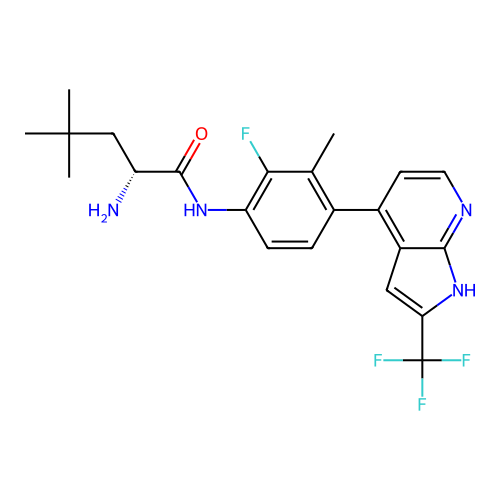
Affinity DataKd: 0.300nMAssay Description:Binding affinity to NanoLuc fused-CITK kinase domain (unknown origin) expressed in HEK293T cells assessed as dissociation constant NanoBRET Target En...More data for this Ligand-Target Pair
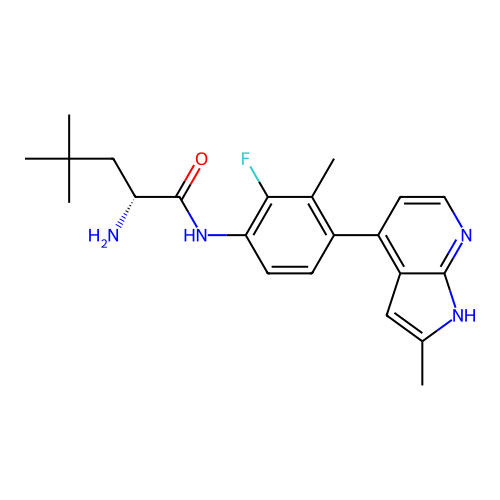
Affinity DataIC50: 3.90nMAssay Description:Inhibition of human STK21 using KKRPQRRYSNVF as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition and measured after 120 mins b...More data for this Ligand-Target Pair
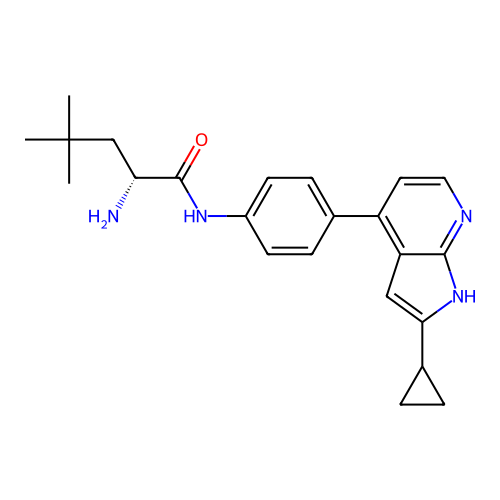
Affinity DataIC50: 5.5nMAssay Description:Inhibition of human recombinant CITK kinase domain assessed as phosphorylation of substrate ULight-ARTKQTARKSTGGKAPRICQLAGC measured after 30 mins by...More data for this Ligand-Target Pair
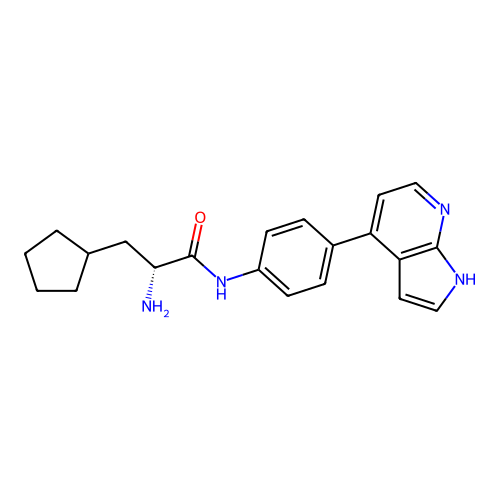
Affinity DataKd: 9.5nMAssay Description:Binding affinity to NanoLuc fused-CITK (unknown origin) expressed in HEK293T cells assessed as dissociation constant NanoBRET Target Engagement Intra...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human recombinant CITK kinase domain assessed as phosphorylation of substrate ULight-ARTKQTARKSTGGKAPRICQLAGC measured after 30 mins by...More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibition of human recombinant CITK kinase domain assessed as phosphorylation of substrate ULight-ARTKQTARKSTGGKAPRICQLAGC measured after 30 mins by...More data for this Ligand-Target Pair
Affinity DataKd: 13nMAssay Description:Binding constant for CIT kinase domainMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Inhibition of human recombinant CITK kinase domain assessed as phosphorylation of substrate ULight-ARTKQTARKSTGGKAPRICQLAGC measured after 30 mins by...More data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibition of human recombinant CITK kinase domain assessed as phosphorylation of substrate ULight-ARTKQTARKSTGGKAPRICQLAGC measured after 30 mins by...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Inhibition of human recombinant CITK kinase domain assessed as phosphorylation of substrate ULight-ARTKQTARKSTGGKAPRICQLAGC measured after 30 mins by...More data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of human recombinant CITK kinase domain assessed as phosphorylation of substrate ULight-ARTKQTARKSTGGKAPRICQLAGC measured after 30 mins by...More data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of human recombinant CITK kinase domain assessed as phosphorylation of substrate ULight-ARTKQTARKSTGGKAPRICQLAGC measured after 30 mins by...More data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Inhibition of human recombinant CITK kinase domain assessed as phosphorylation of substrate ULight-ARTKQTARKSTGGKAPRICQLAGC measured after 30 mins by...More data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Inhibition of human recombinant CITK kinase domain assessed as phosphorylation of substrate ULight-ARTKQTARKSTGGKAPRICQLAGC measured after 30 mins by...More data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Inhibition of human recombinant CITK kinase domain assessed as phosphorylation of substrate ULight-ARTKQTARKSTGGKAPRICQLAGC measured after 30 mins by...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibition of human recombinant CITK kinase domain assessed as phosphorylation of substrate ULight-ARTKQTARKSTGGKAPRICQLAGC measured after 30 mins by...More data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Inhibition of human recombinant CITK kinase domain assessed as phosphorylation of substrate ULight-ARTKQTARKSTGGKAPRICQLAGC measured after 30 mins by...More data for this Ligand-Target Pair