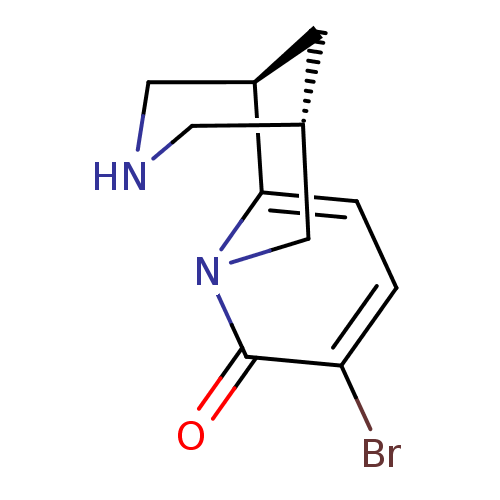
BDBM50329578 (-)-9-Bromocytisine::(1R,5S)-9-Bromo-1,2,3,4,5,6-hexahydro-1,5-methano-pyrido[1,2-a][1,5]diazocin-8-one::3-bromocytisine::CHEMBL365956
SMILES Brc1ccc2[C@H]3CNC[C@H](C3)Cn2c1=O
InChI Key InChIKey=DWDCLEHDNICBMI-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50329578
Found 4 hits for monomerid = 50329578
TargetNeuronal acetylcholine receptor subunit alpha-4/beta-2(Rat)
Ensicaen-Université
Curated by ChEMBL
Ensicaen-Université
Curated by ChEMBL
Affinity DataKi: 0.116nMAssay Description:Displacement of [3H]cytisine from rat alpha4beta2 nAChR in rat brain cell membraneMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-4/beta-2(Rat)
Ensicaen-Université
Curated by ChEMBL
Ensicaen-Université
Curated by ChEMBL
Affinity DataIC50: 0.309nMAssay Description:Displacement of [3H]epibatidine from rat alpha4beta2 nAChRMore data for this Ligand-Target Pair
Affinity DataIC50: 15.6nMAssay Description:Displacement of [3H]epibatidine from alpha3 nAChR in human IMR32 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 401nMAssay Description:Displacement of [125I]alpha-Bungarotoxin from alpha7 nAchR in rat brain cell membraneMore data for this Ligand-Target Pair