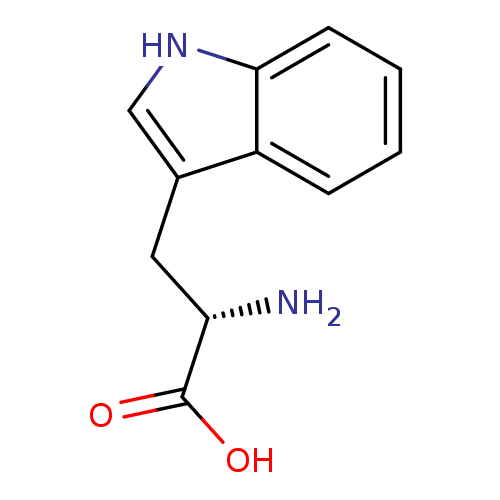
BDBM21974 (2S)-2-amino-3-(1H-indol-3-yl)propanoic acid::CHEMBL54976::L-tryptophan::US11021454, Compound L-trp::US9138393, L-Tryptophan::US9144538, L-Tryptophan
SMILES c1ccc2c(c1)c(c[nH]2)C[C@@H](C(=O)O)N
InChI Key InChIKey=QIVBCDIJIAJPQS-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 16 hits for monomerid = 21974
Found 16 hits for monomerid = 21974
Affinity DataIC50: 2.25E+3nMAssay Description:Inhibition of MPO (unknown origin) using H2O2 as substrateMore data for this Ligand-Target Pair
TargetAlkaline phosphatase, tissue-nonspecific isozyme(Human)
Human Biomolecular Research Institute
Curated by ChEMBL
Human Biomolecular Research Institute
Curated by ChEMBL
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of TNAP by analogous luminescence assayMore data for this Ligand-Target Pair
TargetIntestinal-type alkaline phosphatase(Human)
Human Biomolecular Research Institute
Curated by ChEMBL
Human Biomolecular Research Institute
Curated by ChEMBL
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of IAP by analogous luminescence assayMore data for this Ligand-Target Pair
TargetPhospholipase A-2-activating protein(Human)
Human Biomolecular Research Institute
Curated by ChEMBL
Human Biomolecular Research Institute
Curated by ChEMBL
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of PLAP by analogous luminescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:A commercially available P450-GLO Assay kit (Promega Corporation, Madison Wis.) is used to screen various compounds for CYP3A4A inhibition activity. ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Cytochrome P450 is a large and diverse group of enzymes that catalyze the oxidation of organic substances. Some members of the CYP family contribute ...More data for this Ligand-Target Pair
TargetUracil nucleotide/cysteinyl leukotriene receptor(Human)
Sultan Qaboos University
Curated by ChEMBL
Sultan Qaboos University
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Antagonist activity at human GPR17 expressed in human 1321N1 cells assessed as inhibition of MDL 29,951-induced calcium mobilization after 1 hr by Or...More data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+4nMAssay Description:11.1 Preparation of Reagents and Standard Solutions(1) 75 mM phosphate buffer (PB, pH 7.4): containing KH2PO4 0.0956 g, K2HPO4 0.6946 g, EDTA 1.862 m...More data for this Ligand-Target Pair
TargetLarge neutral amino acids transporter small subunit 1(Human)
University of Nebraska At Kearney
Curated by ChEMBL
University of Nebraska At Kearney
Curated by ChEMBL
Affinity DataIC50: 1.60E+5nMAssay Description:Cis-inhibition of human LAT1 expressed in TREx HEK293 cells assessed as inhibition of [3H]-gabapentin uptake preincubated for 3 mins at 37 degC follo...More data for this Ligand-Target Pair
TargetLarge neutral amino acids transporter small subunit 1(Human)
University of Nebraska At Kearney
Curated by ChEMBL
University of Nebraska At Kearney
Curated by ChEMBL
Affinity DataIC50: 1.60E+5nMAssay Description:Cis-inhibition of human LAT1 expressed in TREx HEK293 cells at 200 uM assessed as inhibition of [3H]-gabapentin uptake preincubated for 3 mins at 37 ...More data for this Ligand-Target Pair
TargetIndoleamine 2,3-dioxygenase 1(Human)
Indo-Soviet Friendship College of Pharmacy (Isfcp)
Curated by ChEMBL
Indo-Soviet Friendship College of Pharmacy (Isfcp)
Curated by ChEMBL
Affinity DataIC50: 4.99E+5nMAssay Description:Inhibition of IDO-1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+5nMAssay Description:In vitro inhibition of recombinant stromelysin catalytic domain.More data for this Ligand-Target Pair
Target2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase(Burkholderia pseudomallei (strain K96243))
Northern Illinois University
Curated by ChEMBL
Northern Illinois University
Curated by ChEMBL
Affinity DataKd: >1.00E+6nMAssay Description:Binding affinity to Burkholderia pseudomallei IspF at pH 7.4 measured by isothermal titration calorimetry methodMore data for this Ligand-Target Pair
Affinity DataKi: 2.80E+6nMAssay Description:Inhibition of human DHFR in presence of DHF and NADPH by UV-vis spectrometry by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 6.50E+6nMAssay Description:Inhibition of human DHFR in presence of DHF and NADPH by UV-vis spectrometry by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.15E+7nMAssay Description:Competitive inhibition of human recombinant TDO expressed in Escherichia coli BL21 using L-tryptophan as substrate by measuring conversion of N-formy...More data for this Ligand-Target Pair
Activity Spreadsheet -- ITC Data from BindingDB
 Found 1 hit for monomerid = 21974
Found 1 hit for monomerid = 21974
ITC DataΔG°: -6.21kcal/mole −TΔS°: 3.90kcal/mole ΔH°: -10.1kcal/mole logk: 3.40E+4
pH: 7.0 T: 25.00°C
pH: 7.0 T: 25.00°C