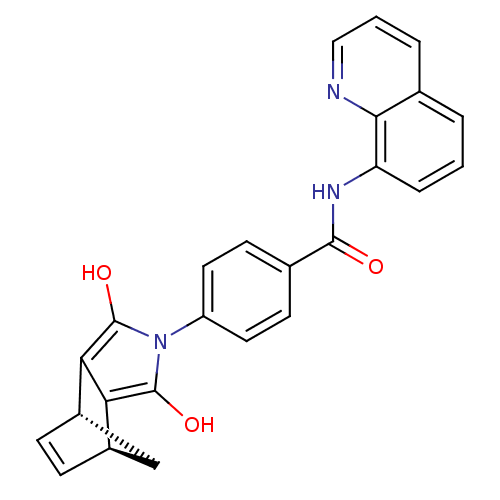
BDBM50294835 4-((1S,2S,6R,7R)-3,5-Dioxo-4-aza-tricyclo[5.2.1.0*2,6*]dec-8-en-4-yl)-N-methyl-N-quinolin-8-yl-benzamide::CHEMBL551505::CHEMBL562310::N-(Quinolin-8-yl)-4-(endo-4-aza-3,5-dioxotricyclo[5.2.1.02,6]oct-8-en-4-yl)benzamide, 1
SMILES Oc1c2[C@H]3C[C@H](C=C3)c2c(O)n1-c1ccc(cc1)C(=O)Nc1cccc2cccnc12
InChI Key InChIKey=OGYSABKWWQZOIU-CALCHBBNSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50294835
Found 2 hits for monomerid = 50294835
Affinity DataIC50: >8.50E+4nMAssay Description:Inhibition of PARP1 (unknown origin) using histone as substrate after 1 hr by luminescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: >1.70E+5nMAssay Description:Inhibition of PARP2 (unknown origin) using histone as substrate after 1 hr by luminescence assayMore data for this Ligand-Target Pair