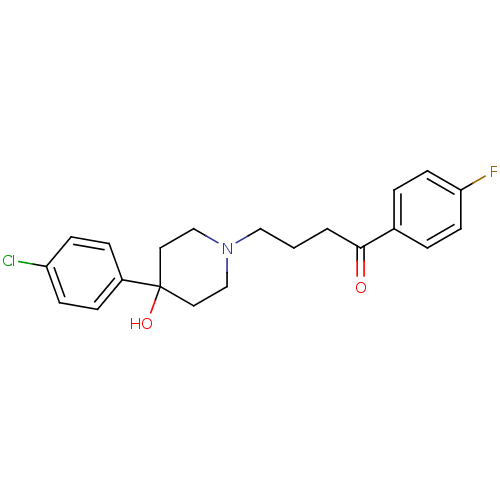
BDBM21398 4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1-(4-fluoro-phenyl)-butan-1-one;propionate(HCl)::4-[4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl]-1-(4-fluorophenyl)butan-1-one::CHEMBL54::CHEMBL545608::Haloperidol::Haloperidol, 1
SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1
InChI Key InChIKey=LNEPOXFFQSENCJ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 23 hits for monomerid = 21398
Found 23 hits for monomerid = 21398
Affinity DataKi: 384nMAssay Description:In vitro binding affinity towards Histamine H1 receptor of rat frontal cortex homogenate by using radioligand [3H]pyrilamineMore data for this Ligand-Target Pair
Affinity DataKi: 384nMAssay Description:Half-maximal inhibition of [3H]pyrilamine binding to Histamine H1 receptor in rat frontal cortex homogenateMore data for this Ligand-Target Pair
Affinity DataKi: 398nMAssay Description:Displacement of [3H]pyrilamine from histaminergic H1 receptor guinea pig cerebellumMore data for this Ligand-Target Pair
Affinity DataKi: 440nMAssay Description:Displacement of [3H]pyrilamine from human H1 receptor by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 440nMAssay Description:Binding affinity to human cloned histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 440nMAssay Description:Displacement of [3H]Pyrilamine from human histamine H1 receptor by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 730nMAssay Description:Binding affinity towards human histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2.10E+3nMAssay Description:Compound was tested for the binding affinity against rat cortical H1 receptor by Radio ligand [3H]-pyrilamine binding assay.More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+3nMAssay Description:Displacement of [3H]mepyramine from H1R in rat brainMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+3nMAssay Description:Inhibitory binding of [3H]-mepyramine to histamine H1 receptors in rat brain membranesMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+3nMAssay Description:Binding affinity towards histamine H1 receptor from rat brain membranes using [3H]-mepyramine as radioligandMore data for this Ligand-Target Pair