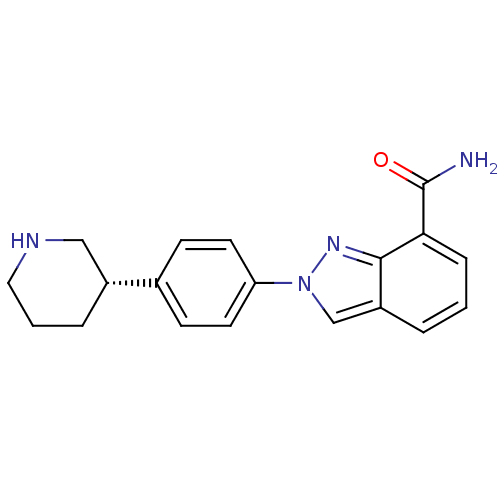
BDBM50316226 (S)-2-(4-(piperidin-3-yl)phenyl)-2H-indazole-7-carboxamide::CHEMBL1094636::MK-4827::Niraparib
SMILES NC(=O)c1cccc2cn(nc12)-c1ccc(cc1)[C@@H]1CCCNC1
InChI Key InChIKey=PCHKPVIQAHNQLW-CQSZACIVSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50316226
Found 2 hits for monomerid = 50316226
TargetProtein mono-ADP-ribosyltransferase PARP3(Homo sapiens (Human))
Health & Science University
Curated by ChEMBL
Health & Science University
Curated by ChEMBL
Affinity DataIC50: 295nMAssay Description:Inhibition of full length recombinant human His6-tagged PARP3 expressed in Escherichia coli BL21(DE3) preincubated for 15 mins followed by biotinylat...More data for this Ligand-Target Pair
TargetProtein mono-ADP-ribosyltransferase PARP3(Homo sapiens (Human))
Health & Science University
Curated by ChEMBL
Health & Science University
Curated by ChEMBL
Affinity DataIC50: 296nMAssay Description:Inhibition of full length recombinant human His6-tagged PARP3 expressed in Escherichia coli BL21(DE3) preincubated for 15 mins followed by biotinylat...More data for this Ligand-Target Pair