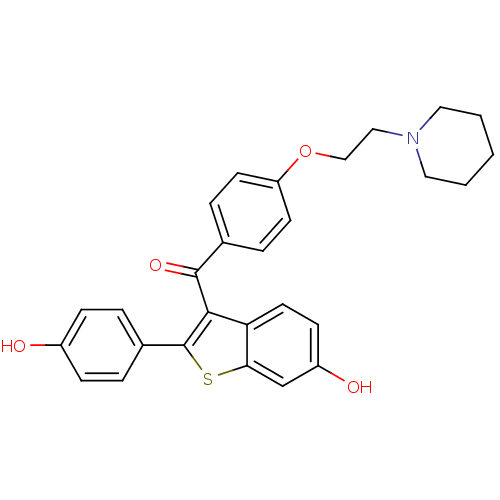
BDBM19441 2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethoxy]phenyl}carbonyl)-1-benzothiophen-6-ol::CHEMBL81::Evista::Keoxifene::RALOXIFENE HYDROCHLORIDE::Raloxifene::Raloxifene (7)::Raloxifene, 6::[6-hydroxy-2-(4-hydroxyphenyl)-1-benzothiophen-3-yl]-[4-(2-piperidin-1-ylethoxy)phenyl]methanone::cid_11071264
SMILES Oc1ccc(cc1)-c1sc2cc(O)ccc2c1C(=O)c1ccc(OCCN2CCCCC2)cc1
InChI Key InChIKey=GZUITABIAKMVPG-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 27 hits for monomerid = 19441
Found 27 hits for monomerid = 19441
Affinity DataKi: 0.0300nMAssay Description:Antagonistic activity against estrogen receptor alpha in presence of 0.1 nM estradiolMore data for this Ligand-Target Pair
Affinity DataKi: 0.220nMAssay Description:Ability to displace [3H]-17-beta-estradiol from Estrogen receptor alpha by scintillation proximity assay.More data for this Ligand-Target Pair
Affinity DataKi: 0.370nM ΔG°: -12.7kcal/mole EC50: 4.32nMpH: 7.5 T: 2°CAssay Description:The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin...More data for this Ligand-Target Pair
Affinity DataKi: 0.370nMAssay Description:Displacement of [3H]estradiol from ERalpha after 4 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 0.380nMAssay Description:Binding affinity to ERalpha ligand binding domainMore data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Binding affinity for estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataKi: 0.430nMAssay Description:Binding affinity to ERalpha (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 0.870nMAssay Description:Uncompetitive type inhibition of human AOX assessed as inhibition constant using phthalazine as substrate preincubated for 30 mins followed by substr...More data for this Ligand-Target Pair
Affinity DataKi: 0.870nMAssay Description:Uncompetitive type inhibition of human AOX assessed as inhibition constant using vanillin as substrate by HPLC-MS analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.870nMAssay Description:Uncompetitive type inhibition of human AOX assessed as inhibition constant using nicotine-1(S)-iminium ion as substrate incubated for 2 mins by HPLC-...More data for this Ligand-Target Pair
Affinity DataKi: 0.870nMAssay Description:Uncompetitive inhibition of human liver cytosolic aldehyde oxidase using phthalazine as substrate assessed as enzyme-substrate complex by Lineweaver-...More data for this Ligand-Target Pair
Target3-beta-hydroxysteroid-Delta(8),Delta(7)-isomerase(Homo sapiens (Human))
University Of Innsbruck
Curated by ChEMBL
University Of Innsbruck
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Affinity for human EMP expressed in ERG2 deficient strain of Sacchromyces cerevisiae using [3H]ifenprodil or (+)-[3H]pentazocine as radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Displacement of [3H]estradiol from full length biotinylated human ERalpha by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.30nMAssay Description:Competitive inhibition of human liver cytosolic aldehyde oxidase using DACA as substrate assessed as free enzyme by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.74nM ΔG°: -11.6kcal/mole EC50: 4.32nMpH: 7.5 T: 2°CAssay Description:The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin...More data for this Ligand-Target Pair
Affinity DataKi: 2.74nMAssay Description:Displacement of [3H]estradiol from ERbeta after 4 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 4.30nMAssay Description:Binding affinity for estrogen receptor betaMore data for this Ligand-Target Pair
Affinity DataKi: 4.30nMAssay Description:Binding affinity to ERbeta (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 4.5nMAssay Description:Antagonistic activity against estrogen receptor beta in presence of 0.1 nM estradiolMore data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Ability to displace [3H]-17-beta-estradiol from Estrogen receptor beta by scintillation proximity assay.More data for this Ligand-Target Pair
Affinity DataKi: 23nMAssay Description:Displacement of [3H]estradiol from full length biotinylated human ERbeta by scintillation proximity assayMore data for this Ligand-Target Pair
TargetSigma non-opioid intracellular receptor 1(Homo sapiens (Human))
University Of Innsbruck
Curated by ChEMBL
University Of Innsbruck
Curated by ChEMBL
Affinity DataKi: 38nMAssay Description:Affinity for sigma receptor type 1 of guinea pig using [3H]ifenprodil or (+)-[3H]pentazocine radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 66nMAssay Description:Affinity for ERG2 of Sacchromyces cerevisiae using [3H]ifenprodil or (+)-[3H]pentazocine radioligandMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2B(Homo sapiens (Human))
University Of North Carolina At Chapel Hill
Curated by ChEMBL
University Of North Carolina At Chapel Hill
Curated by ChEMBL
Affinity DataKi: 69nMAssay Description:Displacement of [3H]LSD from human cloned 5HT2B receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 6(Homo sapiens (Human))
University Of North Carolina At Chapel Hill
Curated by ChEMBL
University Of North Carolina At Chapel Hill
Curated by ChEMBL
Affinity DataKi: 750nMAssay Description:Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells after 1.5 hrs by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 9.90E+3nMAssay Description:Inhibition of CYP3A4 in human liver microsomesMore data for this Ligand-Target Pair
Affinity DataKi: 9.90E+3nMAssay Description:Mechanism based inhibition of human cytochrome P450 3A4 measured by testosterone 6-beta hydroxylationMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)