BDBM124951 US8765972, 4
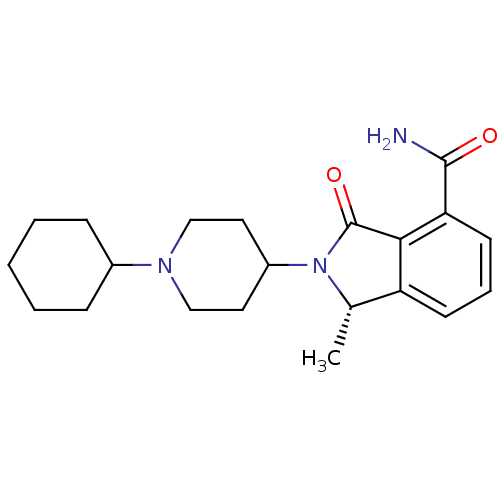
SMILES C[C@@H]1N(C2CCN(CC2)C2CCCCC2)C(=O)c2c1cccc2C(N)=O
InChI Key InChIKey=OYGLTKXMFGWXJT-AWEZNQCLSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 124951
Found 9 hits for monomerid = 124951
Affinity DataKd: 10nMAssay Description:Affinity evaluation of the tested compounds and their selectivity with respect to the different PARP isoforms of interest was assessed in a displacem...More data for this Ligand-Target Pair
Affinity DataKd: >1.00E+4nMAssay Description:Displacement of fluorescent probe from human recombinant His-tagged TNKS1 catalytic domain (1091-1325 residues) by fluorescent polarization assayMore data for this Ligand-Target Pair
Affinity DataKd: >1.00E+4nMAssay Description:Displacement of fluorescent probe from human recombinant His/GST-tagged PARP3 by fluorescent polarization assayMore data for this Ligand-Target Pair
Affinity DataKd: <30nMAssay Description:Displacement of fluorescent probe from human recombinant full length His-tagged PARP1 by fluorescent polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: >3.10E+4nMAssay Description:Inhibition of CYP2B6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKd: 16nMAssay Description:Binding affinity to human recombinant His/GST-tagged PARP1 catalytic domain (655-end residues) by surface plasma resonance methodMore data for this Ligand-Target Pair
Affinity DataKd: >1.00E+4nMAssay Description:Displacement of fluorescent probe from human recombinant full length His-tagged PARP2 by fluorescent polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:Inhibition of PARP1 in human HeLa cells assessed as reduction in H2O2-induced PAR formation incubated for 30 mins followed by H2O2 addition for 15 mi...More data for this Ligand-Target Pair
Affinity DataIC50: >3.10E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)