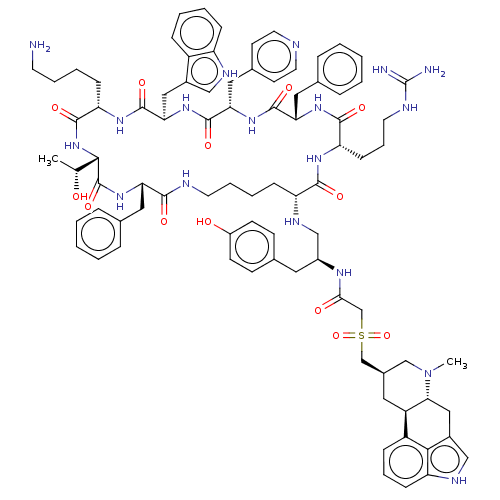
BDBM144762 US8952128, 21
SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccncc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](CCCCNC(=O)[C@H](Cc2ccccc2)NC1=O)NC[C@H](Cc1ccc(O)cc1)NC(=O)CS(=O)(=O)C[C@@H]1C[C@H]2[C@@H](Cc3c[nH]c4cccc2c34)N(C)C1
InChI Key InChIKey=SBZRVZDPNGGKPV-UHFFFAOYSA-N
Data 2 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 144762
Found 2 hits for monomerid = 144762
Affinity DataKi: 0.0200nM ΔG°: -14.6kcal/molepH: 7.6 T: 2°CAssay Description:Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMpH: 7.6Assay Description:The affinity of a test compound for the human dopamine receptor subtype hDRD2 was determined by radioligand binding assays in CHO-K1 cells stably tra...More data for this Ligand-Target Pair