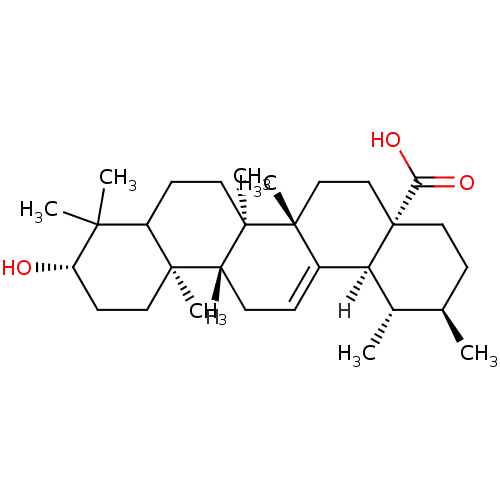
BDBM23197 (1S,2R,4aS,6aS,6bR,10S,12aR,12bR,14bS)-10-hydroxy-1,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylic acid::US11660306, Example Ursolic acid::Ursolic Acid, 10::Ursolic acid (UA)::pentacyclic triterpene compound 10
SMILES [H][C@@]12[C@@H](C)[C@H](C)CC[C@@]1(CC[C@]1(C)C2=CC[C@]2([H])[C@@]3(C)CC[C@H](O)C(C)(C)C3CC[C@@]12C)C(O)=O
InChI Key InChIKey=WCGUUGGRBIKTOS-JJWDWEPMSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 23197
Found 4 hits for monomerid = 23197
TargetTyrosine-protein phosphatase non-receptor type 1 [1-405](Human)
Cold Spring Harbor Laboratory
US Patent
Cold Spring Harbor Laboratory
US Patent
Affinity DataKi: 900nMAssay Description:Glucose in tail blood was measured using a glucometer (One-Touch Basic; Lifescan, CA). For glucose tolerance tests (GTTs), mice were fasted for 10 ho...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1 [1-321](Human)
Cold Spring Harbor Laboratory
US Patent
Cold Spring Harbor Laboratory
US Patent
Affinity DataKi: 2.00E+3nMAssay Description:Glucose in tail blood was measured using a glucometer (One-Touch Basic; Lifescan, CA). For glucose tolerance tests (GTTs), mice were fasted for 10 ho...More data for this Ligand-Target Pair
Affinity DataIC50: 9.00E+3nMpH: 7.2 T: 22°CAssay Description:The activity of the compounds is determined by measuring the inhibitory effect of the compounds in the direction of glycogen synthesis, the conversio...More data for this Ligand-Target Pair
Affinity DataIC50: 3.20E+3nMpH: 6.5 T: 30°CAssay Description:Briefly, the enzymatic activity of the PTP1B catalytic domain was determinedat 30°C by monitoring the hydrolysis of pNPP. Dephosphorylation of pNPP g...More data for this Ligand-Target Pair